Citation: Sunil A and Somya A. Lean Six Sigma for Medical Device Excellence and Regulation. Adv Pharmacol Clin Trials 2016, 1(2): 000111.
*Corresponding author: Sunil Aggarwal, Master Black Belt Lean Six Sigma, New York, USA, Tel: 1-717-622-1264; Email: drsunilaggarwal@gmail.com
Medical device industry is one of the fastest growing industries in US with over $80 billion market. The industry makes medical products ranging from simple glucometers to computer managed surgical gadgets, from implants to artificial organs. There are new products being launched in the market on a daily basis. Lean Six Sigma plays a key role in quality management throughout the medical device product life cycle and helps to achieve desired end results.
Keywords: Medical device; Regulation; FDA, Quality; Lean six Sigma
Abbreviations: FDA: Food and Drug Administration; ISO: International Organization for Standardization; SWOT: Strengths Weakness Opportunity Threats; CAPA: Corrective Actions Preventive Actions
The expertise in manufacturing a medical device is from its start and most medical device industries realize this or have begun to realize this in the product life cycle. The regulatory compliance from FDA or ISO 13485 has mandated strict compliance guidelines through their Quality System Regulations (QSR) and this is definitely of advantage to recognize the importance of quality and rigid regulations right from the incubation to the growth of a medical product and bring out a competitive edge in the medical device industry [1-6]. This incorporates operational excellence and best achieved by elimination of waste and reducing cost, yet maintaining quality and adhering to strict regulations. Lean helps to create improved work approach and generate more revenues [1].
MethodologyMedical Device Company AB (MDCAB) is excited to get a new medical product development launch. They have basic engineering tools in place for the design and development with acceptance-sampling plans and they have also identified equipment qualification document. MDCAB also has release forms for product/batch, once the technology transfer is done apart from their design and development of the medical device. The company has a history of successive rejection from regulatory authority due to non -compliance of regulatory and quality compliance. Many years of product development and millions of dollars were wasted due to these rejections [2]. MDCAB has initiated this product with the help of an experienced lean six sigma team with the hope of reducing waste and quality management and ultimately getting the product approved by regulatory authorities.
The Lean six sigma chief noticed broad areas of concern, right away and formed three teams in the area of operational excellence with documentation, quality design and approach to the regulatory authority. These three teams had experienced lean trained professionals and worked on various departments in MDCAB that were involved with the product. The final end product of all these three teams were to be analyzed to get the desired results; save time by reducing waste; save money by timely action; get right results by achieving targets.
Process ImportanceAll the three teams used DMAIC as the standard tool to study the requirements for effective improvement, better quality designs, better medical device product capabilities and competencies and in general focused themselves on achieving compliance with product design control or after market analysis. All the three teams did a preliminary SWOT analysis within their respective units about the novel project. The strengths and weaknesses that emerged were predominantly inherent to functional capabilities of the working system and that the weaknesses had to be turned into strengths by external opportunities and minimizing threats. The threats were the immediate failure to achieve end target in the prior two devices and also possible threats coming in the future in the system for new projects, including this one.
Based on multiple surveys, the Voice of customers (VoC) was very crucial to the outcome as the strategic initiatives, tactical approach to product, market, services, and detailed customer attributes. The need for communicating VoC was inherent to the success of lean initiative.
Eventually, an analysis of regulatory mechanisms was done by these three teams as one team, based on their product process input (Figure 1).
DFSS- Medical Device experience
Identify phase
There was an air of excitement at the launch of a new project for this novel medical device and seemed interested to know as to how soon the project would initiate, but there was also a palpable fear in the team for a failure to achieve end result, as happened on two prior instances. The MDCAB team was aware of lean six sigma involvement, this time, and had a discussion last week about the rationale for such a quality management team. The medical device development team were aware that the Voice of customer is most important as just being good is not enough; it has to be a customer satisfaction based superior innovative technology, based on their SIPOC analysis (Figure 2).
Problem definition
Team MDCAB is working on an innovative design to manufacture a novel medical device for an artificial organ. They have had two failures in the past. There are engineers who do their job with utmost perfection; there are design development professions and testers, but there is no time schedule that the device development goes through a department. Virtually no time is left for reanalysis and discussion on simple “makes sense” questions before going into complexities of device manufacturing.
MDCAB is not fully aware about internal and external compliance audits and no professional mechanisms to display that the company has a quality system to demonstrate safety and effectiveness of the medical devices they produce [5].
Characterize phase
The prior gut based experiences of doing things in “this” way were much prevailing in all the teams, across the board. Some common questions during product development that was to be measured were:
a) Is there a superior authority in MDCAB that can say if the design and development process is correct and the best?
b) Does the design answer the basic needs of the customers?
c) Is there professional team of experts providing specification to the design and development?
d) Were these professionals aware of potential development possibilities when designs were developed?
e) Is there a mechanism in place to modify the specifications?
f) How would such a device mean profitability to MDCAB?
g) Did anyone keep in mind of time frames?
h) Did the process of the new device development go through the phases of concept acknowledgement, design for device preparation and production process?
i) Are there after marketing robust surveys from the customers of use?
j) Is there a team of regulatory compliance trained professionals especially for regulatory audits with international regulators and CAPA/Nonconformance reports?
Optimize phase
The three teams met to analyze as to how the failure can be avoided and achieve operational excellence. The analysis of the teams was summarized as follows:
A. The design team must begin to work on the concept of design and maximally influence at that stage to get the final outcome with complete understanding of the customer needs and MDCAB capabilities.
B. Concept of design preparation included VoC analysis (demographics, customer questions preparation, sample surveys and data collection) and then organizing this analysis into a “shape” and finally work on its analysis.
C. Critical to quality analysis of customer needs, and speedy assimilation of design assembly and manufacturing with the available or integrated engineering resources.
D. A visit to 12 cardiac surgeons was done to map out their needs and add more meaning to VoC.
E. An affinity diagram was prepared that highlights VoC and details customer preferences (Figure 3).
F. It is known that stagnation of design assembly and manufacturing can slow the pace of development and better decision making, whenever required.
G. Cash drain through slower process can be avoided to achieve better fiscal results.
H. Mock audits were done by trained compliance audit personnel designated rooms and roles assigned to each member.
Certify/Validate phase
The various heads of the teams got together to work on a standardized platform to speed up the process in concept design, design assembly and manufacturing engineering unit and weekly updates to the teams were made and if required a monthly meeting of all the teams to be aware of the development of the new device. This ensured that all the stakeholders in this project of device development had a voice in the process. The Vice President, novel device development, was constantly keeping a track of it.
During this phase best standards were marked as benchmark process of improvisation and were recorded as good business practices [4,6]. CAPA/ regulatory audits are in place now with extensive mock audits. The regulatory compliance verifies prior audit cases and shortcomings therein. Risk management is based on potential risks ascertained throughout (Figure 4).
.This phase appeared to be very important for MDCAB to maintain consistency of quality parameters. All the involved and other employees received a handout of the various quality measurements taken and that they were expected to follow similar practice in their work.
A high level CDP preparation committee has been formed to look into the concept, design and production phase of all future products (Figure 4). Management review meetings take place on schedule with proper agenda.
Results and ConclusionThis medical device was approved by regulatory authorities and was benchmarked in the history of MDCAB as a state of the art device in record time. Superior design will deliver better customer satisfaction through superior product. Regulatory compliance is much more effective now with proper trained professional guiding the way right from design of concept. This generated and saved millions of dollars. This went on to show that lean is an advanced planning tool and the way forward. VoC is the silver lining to success and companies that listen to the customers in their production house have a higher chance of success.
Originality/ValueThis paper is a benchmark and a guide for various medical device companies competing today with an aim to achieve more. This will set standards of operational care and management excellence.
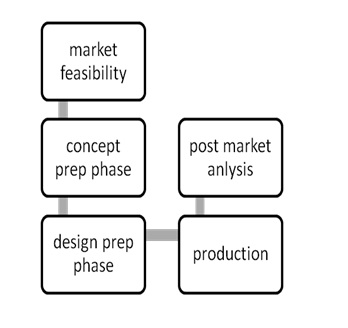
Figure 1: Medical Device Development Process.
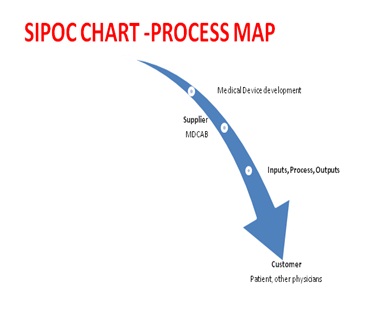
Figure 2: SIPOC Model
Figure 3: Affinity Diagram.
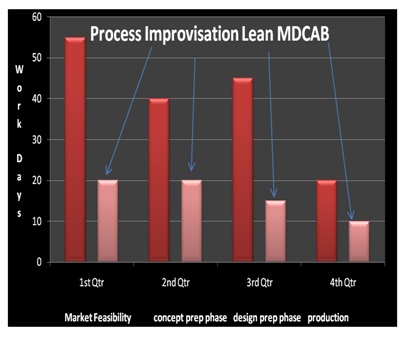
Figure 4: Process Improvisation by Lean