
Citation: Sunil A, et al. Global Regulatory Intelligence for Regulatory Strategy and Good Practice. Adv Pharmacol Clin Trials 2016, 1(3): 000113.
*Corresponding author: Sunil A, MD, MBA, Master Black Belt Lean Six Sigma, New York, USA, Tel: 1-717-622-1264; Email:drsunilaggarwal@gmail.com
Bringing a new medical device or drug has become more challenging than ever in terms of competition, speed of delivery, regulatory monitoring, efficacy and safety and heavily depends on total quality management (TQM), globally. This is all about Good Regulatory Practice, as global regulatory requirements may vary from one country to another. Maximum regulatory compliance for a medical device can prove to be costly and regulatory intelligence would be required for the entire life cycle of the medical device as the regulatory strategy in terms of safety and efficacy. An effective regulatory policy leading to a competitive regulatory strategy is required.
Keywords: Medical device; Regulation; Regulatory intelligence (RI)
Regulatory Intelligence is the process of analysis of data acquired through regulatory information. RI emerged about fifteen years back but is yet to get a firm ground. Many regulatory professionals are not clear about the distinction between regulatory information and regulatory intelligence. Regulatory information is the important step in the process of RI, where we gather the necessary information or data of regulatory strategies to achieve targeted results and get the product out on time. Total Quality management (TQM) pertains to the manufacturing company keeping up with quality management or ISO, US or European agencies requirements for quality. TQM greatly enhances output and streamlines the manufacturing and marketing process [1].
Definitions of RIVarious agencies have defined RI.
The DIA RING“The act of gathering and analyzing publicly available regulatory information. This includes communicating the implications of that information, and monitoring the current regulatory environment for opportunities to shape future regulations, guidance, policy, and legislation”.
The EU RING“Regulatory intelligence is the act of processing targeted information and data from multiple sources, analyzing the data in its relevant context and generating a meaningful output – e.g. outlining risks and opportunities - to the regulatory strategy. The process is driven by business needs and linked to decisions and actions”. In spite of various definitions, it is clear that regulation, and specifically RI, must be transparent, consistent, and measurable. RI should be reproducible and quantifiable in terms of dimension and output.
Regulatory Intelligence: The processThe regulatory information is gathered mostly from regulatory agencies like FDA or EMA websites or other regulatory agencies information mechanisms [2]. Some information is also acquired through thought leaders’ related publications and also from internal communications within the organisation. Information may also be obtained from webinars, seminars and personal interactive information.
Once the key information acquisition takes place, the process of analyses of this acquired regulatory information begins. SWOT (Strengths, Weakness, Opportunities, Threat) analysis is implemented for efficient outcome [3]. The data on regulatory information is ever increasing and is so vast that it becomes mandatory to now retain the relevant information for the purpose of the clinical trial or device development. This means it becomes imperative to filter this information and use the best signal and eliminate added frequencies of noise [4]. Knowledge of one regulatory body does not suffice as regulatory strategies differ across the globe. European Union has different regulations than FDA in US. The whole idea is to gather relevant strategy in terms of prioritization of approach in regulatory approval from various target countries in the least possible time and decrease the cost of overall production [3,5].
Once the regulatory information is filtered, the final step of RI is the formation of a regulatory strategy. Regulatory strategy is developed from the regulatory information and its analyses. The regulatory strategy is continuously being updated as the new regulatory information keeps getting in. This makes regulatory strategy or the strategy document to be a flexible document that requires continuous updates, as and when necessary, based on the new information acquired.
Regulatory Intelligence: Process ImportanceThe regulatory intelligence can advance the medical device life cycle by its significant inputs [6] and begins by gathering regulatory information from various regulatory websites or through conferences, books, journals and other print medium. Regulatory information is also obtained from local chapter meetings and internal information from within the company. FDA issues many warning letter and regulatory blogs could give substantial information.
The next step would be an analysis of this information by key focus on relevant data from the vast data available. The evaluation of this data and the trends and precedents that hare there from various competitors would be done. A gap analysis and SWOT (strengths, weakness, opportunities, and threats) would be necessitated to look for possible shortcomings [5]. The correct interpretation of this data would lead us to proceed towards formulation of a regulatory strategy.
Regulatory strategy is live and flexible as emergence of new regulatory information will become new inputs and cannot be rigid to the regulatory information. Priorities and data integration with the requirements to achieve 510(k) or PMA is necessitated. The reports so generated are disseminated and communicated to all the stakeholders, whether parent manufacturer of part suppliers and are key to achieve targeted result (figure 1).
RI-Need of the dayRegulatory Intelligence is proving to be the accelerating factor for medical device and pharmaceutical industry towards achieving regulatory benchmark. It also establishes a transparency in the system as the strategy document is also visible to the competitors. It helps to bring a better return on investment by optimizing and enhancing acceleration in the system and culture in the organisation. Emergence of complex medical devices and drugs mean better regulatory strategies for the developers. Prospective developments such as 3D printing are bound to pose many questions for regulators in terms of justification of an end product. Continuous update of information of changing trends and legislation with awareness of its impact on global regulations is required by regulators [1].
Various interactive intelligence processes play a key role in the formation of regulatory strategy (Figure 2). Business intelligence information for regulatory strategy and risk analysis of medical devices is working from the regulatory procedures in place in the company. The imperative knowledge of procedures and tasks would define business strategies and environmental and legal intelligence about the regulator competence to perform and the acceptability of regulatory strategies in terms of framework or existing tools.
Competitor intelligence is indeed a necessary source of information to understand if your own regulatory strategy would be successful or not based on a comparative analysis obtained from a competitor strategy [7].
ConclusionInnovation is the pivotal point in globalization of regulatory mechanisms across the globe. Not only is it required by the manufacturing industry but also by the patients. It just not brings regulatory journalism in the associated professionals, but it is required for a platform that would provide all regulatory resources and provides the organization with a stronger presence in a global market. Undoubtedly, regulatory intelligence is a catalyst for growth in the growth dynamics of a company. Regulatory journalism may be the way forward in regulatory strategy guidance, across many nations.
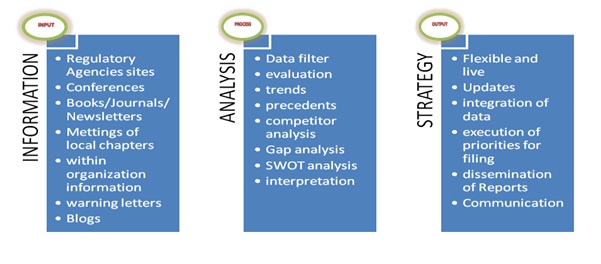
Figure 1: Regulatory intelligence: How to do it?
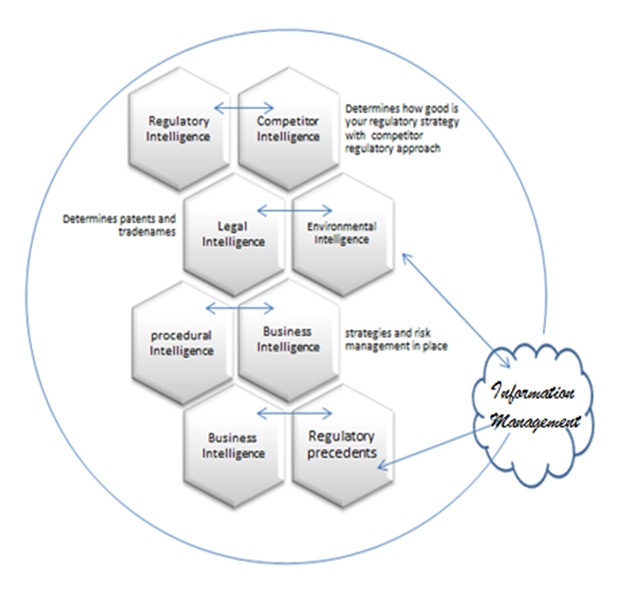
Figure 2: Information management: Feeder to all Intelligence system in the process.