
Citation: Goel V. Marjolin’s Ulcer. Clin Dermatol J 2017, 2(4): 000125.
*Corresponding author: Dr Vipin Goel, Department of Surgical Oncology in Basavatarakam Indo American Hospital, Hyderabad, India, Tel: 9844115040; Email: vipinrgoel@gmail.com
Marjolin’s ulcers are skin cancers developing on preexisting chronic inflammatory skin lesions. Most common histology is squamous cell carcinoma followed by basal cell carcinoma and malignant melanoma. Rarely fibrosarcoma, angiosarcoma, liposarcoma, leiomyosarcoma, osteosarcoma, dermatofibrosarcoma protuberans, malignant fibrous histiocytoma is reported [1]. The incidence of Marjoli’s ulcer is following a constant increasing trend. Marjolin’s ulcers are more common in males. Male to female ratio is 2:1. The Marjolin’s ulcer is a disease of middle and old age. The mean age of presentation being 55 years. But in recent times, more and more young patients are reporting with a Marjolin’s ulcer. The leading aetiology of the ulcer is a post-burn wound. All types of burns: thermal, electrical, chemical and radiation burns can lead to a Marjolin’s ulcer. Other causes being chronic traumatic ulcers, venous ulcers, chronic sinuses of osteomyelitis, pressure sores. Pathogenesis of Marjolin’s ulcer is multifactorial. In the chronic non healing ulcer there is a constant epidermal cell proliferation with immature granulation and epidermal tissue formation. In these immature cells, there is a mutation of p53 and other tumour suppressor genes. The p53 tumour suppressor gene prevents uncontrolled multiplication of cells by repairing damaged DNA or by signalling for apoptosis. If p53 is mutated or lost, appropriate DNA repair and/or apoptosis do not occur. As the cell cycle progresses mutated daughter cell multiplies unchecked, leading to cancer [2]. Another factor postulated is reduced vascularity in the ulcer bed. Reduction in the Langerhans cell population because of reduced vascularity leads to loss of defence forces with inability to withstand carcinogens. There is a time period (Transition time) from onset of ulcer to the transformation of the ulcer into malignancy. Transition time is the period from the onset of injury/ulcer to the development of Marjolin’s ulcer. The transition time is variable, ranging from three months to 63 years. The average transition time is 26 years [3]. Over the years the transition time is getting shorter. If transition time is < 2- 3years, it is called acute Marjolin’s ulcer. The lower limbs are the most common site of Marjolin’s ulcers, constituting almost 50 % of all cases. Other sites in descending order being upper extremities, head and neck and trunk [4].
The most common presentation is a painless, nonhealing ulcer [5]. A treating physician requires a high index of suspicion to differentiate a non healing ulcer from a Marjolin’s ulcer. Clinicians who have not seen or treated Marjolin’s ulcer in the past may find it difficult to differentiate between these two conditions. Onset of new ulcer on healed scar is second most common presentation. It's not difficult to make a diagnosis in patients with the development of a new ulcer on scar tissue. The ulcer may be associated with bleeding or persistent discharge. Rarely, patients can present with swelling in the lymph node drainage area. The clinician should thoroughly examine the primary limb for any ulcer/lesion if patient presents with lymph nodal mass. Squamous cell carcinoma resulting from Marjolin’s ulcer has a greater tendency to metastasize to lymph nodes once it enters the skin outside of the scar as compared to non-Marjolin’s squamous cell carcinoma. Very rarely, patients may present with symptoms of lung metastasis like cough, chest pain and hemoptysis. Relevant past history about lesion like skin disorder, burns, venous ulcers, discharging sinuses of osteomyelitis, pressure sores helps in clinching diagnosis. In late stages cancer can involve bone. In such scenario, the presentation may be with pathologic fractures or constant pain because of periosteal inflammation [6].
On examination, the Marjolin’s ulcer is either flat, indurated, infiltrative type or exophytic polypoidal type [7]. Infiltrating type is having a poorer prognosis than exophytic type. On inspection of ulcer, the edge is everted and the floor is covered with un-healthy granulation tissue (Figure 1). On palpation, the ulcer is painless with indurated floor and base. The surrounding skin is usually normal on inspection and palpation. Examination of the draining lymph node basin may show enlarged lymph nodes. Lymphadenopathy if present is either because of infection of the ulcer or lymph node metastasis. The tender, soft, and oval nodes are more suggestive of the non-malignant lymphadenopathy. Painless, hard, and round nodes are suggestive of lymph node metastasis.
The lung is most common distant metastatic site. The lungs should always be auscultated to rule out added sounds. Liver, brain, kidney and other distant metastases can also occur but very rarely.
In a suspected case of Marjolin’s ulcer diagnosis is confirmed by biopsy [8]. Usually an incisional biopsy is preferred over excisional biopsy. Biopsy should be repeated in suspicious lesion when an incision biopsy report is negative, but suspicion of Marjolin’s ulcer is higher. In proven cases of Marjolin’s ulcer ultrasound of the draining area should be done to see lymph node status. Characterization of enlarged nodes should be done differentiating benign vs malignant nodes. X- ray chest should be done to rule out lung metastasis.
Treatment is based on stage of disease (Figure 2). Wide local excision should be done for disease localized to primary site where the lesion is free of underlying bone and without lymph node and lung metastasis. Wide excision is curative in this group of patients. A margin of 1-2 cm is usually considered curative. Reconstruction of defect required either split skin grafts or flap reconstruction based on the soft tissue defect [9]. Amputation is required in extremity lesions with bone involvement by cancer and invasion of major neurovascular structures by cancer [10]. Before morbid surgery like amputation is undertaken, the bone erosion by tumour should be differentiated from infection (osteomyelitis). In cases with clinically negative lymph nodes and imaging showing benign nodes, patients should be kept on regular follow up without addressing lymph nodes. Adequate lymphadenectomy is required for cases with positive nodes clinically/radiologically.
Radiation therapy is mainly indicated for inoperable regional lymph node metastasis and > two positive lymph nodes after regional lymph node dissection [11]. Chemotherapy is indicated for metastatic disease. In metastatic disease, chemotherapy is only palliative. Surgery may be required in metastatic stage to palliate symptoms of bleeding and fungation.
Follow up should be aggressive in first 2 year as maximum recurrence occurs within first 2 years. In the first 2 year patient should be called every 3 months for detailed history and physical examination. Both local and regional lymph node examination should be done at every visit. In symptomatic patient and patients with suspicious examination finding imaging should be done. Imaging primarily consists of ultrasound of regional lymph nodes and X-ray chest [12]. After first 2 years patient should be followed every 6 months and after 5 years he should be followed yearly. In patients with recurrent lesions wide re-excision should be done. Re excision in such cases is usually curative.
Tumor type, location, and stage of disease decides prognosis. Marjolin's ulcers are more aggressive than other forms of non-Marjolin’s skin cancer when comparing stage by stage. The overall metastatic rate of Marjolin's ulcers is approximately 27% [4]. The overall 3 year survival for patients with Marjolin's ulcer is 65%- 75%.
Marjolin’s ulcers are aggressive tumours with poor prognosis. Early diagnosis with prompt and adequate treatment reduces mortality. After treatment, the patient should be kept on close follow-up to detect recurrence in early stage.
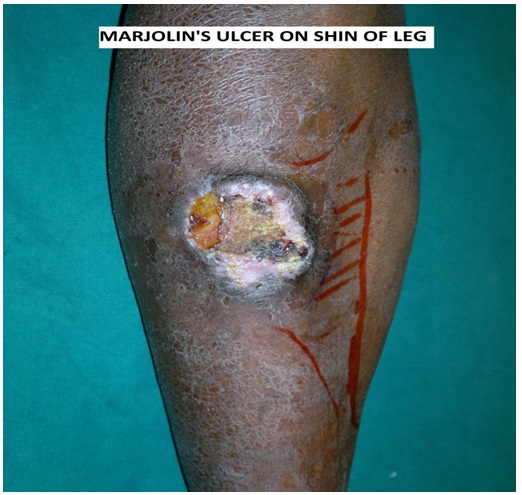
Figure 1: Marjolin’s Ulcer on shin of leg.
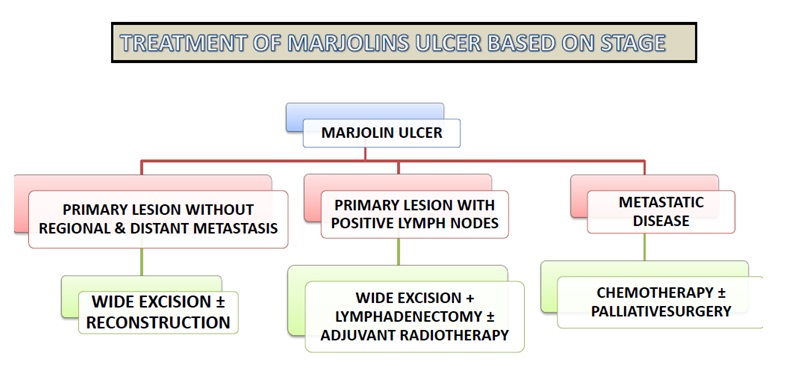
Figure 2: Treatment of Marjolin’s ulcer based on stage.