
Citation: Deepak K et al. In-Vitro Activity of Teicoplanin against Clinical MethicillinResistant Staphylococcus aureus Isolates. J Microbiol Biotechnol, 2017, 2(1): 000112.
*Corresponding author: Dr. Deepak Kumar, Assistant Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India, Tel: +91-9451354332; Email: deepak.mln30@gmail.com
Aim: To evaluate the in vitro activity of teicoplanin against clinical methicillin resistant Staphylococcus aureus isolates was the aim of this study
Methods and Material: A total of 62 previously confirmed MRSA isolates were included in this study. Susceptibility testing and result interpretation of isolates to teicoplanin was performed by the E test and disk diffusion method as per Clinical and Laboratory Standards Institute (CLSI) M100-S25 recommendations.
Results: Teicoplanin appears to exhibit excellent in vitro activity against the MRSA isolates.
Conclusion: The high in vitro susceptibility to teicoplanin in this population and fewer adverse drug effects, teicoplanin may be considered a useful first line antibiotic agent in the treatment of infections caused by MRSA.
Keywords: Teicoplanin; MRSA; E Test; Disk Diffusion Method
Methicillin-resistant Staphylococcus aureus (MRSA) are among the most prevalent pathogen responsible for hospital acquired infections [1]. MRSA infection is hard to treat as methicillin resistance in general is associated with other class of drugs like macrolides, lincosamides, aminoglycosides, quinolones, and trimethoprimsulfamethoxazole. The treatment option for this multidrug resistance organism is limited to glycopeptide antibiotics like vancomycin and teicoplanin. Vancomycin usage is presently a concern to clinician because of decreased efficacy despite MIC value in susceptible range [2]. Other factors restricting its use are MIC creep phenomenon and side effects like ototoxicity, and nephrotoxicity [3]. Teicoplanin on the other hand hold the promise to be a useful alternative in cases where vancomycin is clinically ineffective and in cases where use of vancomycin is curtailed due to adverse drug affects [4,5].
Teicoplanin is a naturally occurring lipoglycopeptide that kills Gram-positive cocci by inhibiting peptidoglycan biosynthesis by binding to the D-alanyl-D-alanine termini of peptidoglycan intermediates, thus disrupting late-stage steps in cell wall biosynthesis [6]. This drug shows a great therapeutic effect to MRSA. With the constant change in the epidemiology of MRSA throughout regions and countries a variation in its drug-resistance patterns is reported [7]. Unfortunately no current resistance data from our area are available. It is thus mandatory to monitor the trends of resistance to teicoplanin in the MRSA causing infection. The aim of this study was evaluate the in vitro teicoplanin activity against clinical MRSA isolates.
Material and MethodsThe study was carried out in the Department of Microbiology, Institute of Medical Science Banaras Hindu University, Varanasi U.P. India. The total duration of study is one year; period extends from July 2015 to June 2016. A total of 62 non-duplicate MRSA isolates from various clinical specimen like pus, blood, urine, tracheal aspirates, sputum, central venous catheters tips, CSF and other sterile body fluids were randomly selected. MRSA isolates were identified by standard microbiological techniques. Methicillin resistance was confirmed by using cephoxitin disc (30 µg Himedia labs) After isolation and identification, the MRSA strains were kept at -200C in peptone/glycerol (30% w/v), and before teicoplanin susceptibility testing, the strains were purified twice on blood agar plates.
Susceptibility testing of the isolates to teicoplanin was performed by the disk diffusion method as per Clinical and Laboratory Standards Institute (CLSI M100-S25) [8]. The MIC values were obtained by E-test, which was performed as per manufacturer's instructions using gradient strips of teicoplanin (Biomerieux France). The disc diffusion test was performed with cation adjusted Mueller-Hinton agar (Himedia Labs) as testing media. Teicoplanin disks of content 30µg (BD USA) was used. The inoculated plates were incubated in ambient air at 37°C for 16 to 18 h. MRSA strain ATCC 43300 was used as control strains, zone of inhibition and MIC values obtained was interpreted by using CLSI M100-S25 (2015) breakpoints. The isolate was considered susceptible to teicoplanin if the zone of inhibition ≥ 14mm; intermediate if zone of inhibition ≤ 10mm, and intermediate if zone size is in range 11-13mm. The MIC breakpoints for teicoplanin as pre CLSI was (< 8 µg/ml, S; 16 µg/ml, I; > 32 µg/ml, R).
ResultsA total of 62 clinical MRSA isolates were included in this study. Table: 1 shows distribution of MRSA among various clinical samples. From the above table it can be said the highest number of methicillin resistant strains were obtained from pus (38.7 %) followed by blood (16.1 %) and sputum (12.9 %).
The main finding of our study is that teicoplanin exhibits excellent antimicrobial activity against MRSA isolates as none of the isolates obtained was resistant to teicoplanin. The distribution of zone of inhibition is soon in table 2. The zone size of inhibition by disc-diffusion method ranges from 14-18mm with median value of 15.7mm. Majority of the isolate (53.2 %) had zone of inhibition > 16mm. The distribution of MIC values and MIC range for teicoplanin is shown in table 3. The MIC values obtained by E-test ranges from 0.38-2.00 µg/ml with a mean value of 1.00 µg/ml. For majority of isolate (69.3 %) the MIC values were < 1 µg/ml. After applying the CLSI interpretative criteria all the MRSA isolates were found susceptible to teicoplanin by both the methodology. Thus a 100% concordance between the results of E-test and DD method was found.
DiscussionMethicillin-resistant Staphylococcus aureus (MRSA) is causing a wide variety of human diseases and hence increasing burden on healthcare resources. Vancomycin has been the only uniformly effective treatment for MRSA infections in India, and other glycopeptides not being commonly used. The role of vancomycin in the treatment of MRSA has been questioned and debated due to the spread of vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA) [9,10]. Teicoplanin, a glycopeptide class antibiotic is used intravenously or intramuscular to treat serious MRSA infections. In our study, we examined the susceptibility of teicoplanin by disc-diffusion and E-test. Few studies have reported that detection of teicoplanin resistance by conventional disc diffusion methods is difficult because of limited diffusion of its large molecule in agar [11]. This study did not find and limitations of disc susceptibility testing for MRSA with teicoplanin. Both methods provided similar results and we did not find any resistance to teicoplanin in our tested MRSA isolates.
The data presented in this study support the findings of studies performed worldwide which says that the number of MRSA resistant to teicoplanin is very low [12]. Several studies have demonstrated the relationship between teicoplanin MICs values and treatment outcome in patients with MRSA infections. A study by Charlesworth et al showed that a higher teicoplanin MIC was associated with a lower survival rate in critically ill patients [13].The present study confirms the good in vitro activity of teicoplanin against MRSA as the mean MIC values of teicoplanin in our study was 1.00 mg/L. The high in vitro susceptibility of the isolates tested to teicoplanin and low resistance prevalence may be related to its restricted use for the treatment of MRSA infections. Teicoplanin thus, may be considered a promising treatment option for the treatment of MRSA infections in our region.
ConclusionBased on the above results, it can be concluded that teicoplanin, is a good choice for the treatment against bacterial infections caused by methicillin resistant Staphylococcus aureus (MRSA). The comparative analysis demonstrates that disc-diffusion and E-test provide similar results of susceptibility. Thus, for susceptibility testing disc diffusion can be easier and economical option as compared to E test.
AcknowledgementThe authors would like to thanks Mr. Atul Kumar Awasthi area manager SANOFI for providing Teicoplanin Disc and E strips for this study.
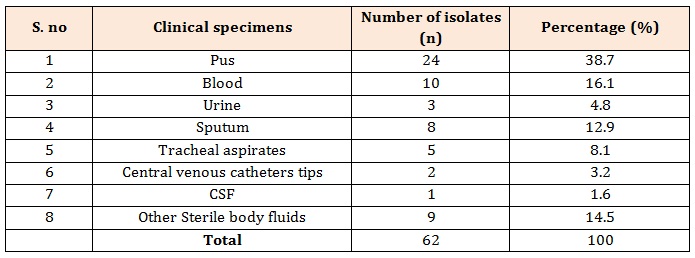
Table 1: Distribution of MRSA isolates from various clinical specimens.
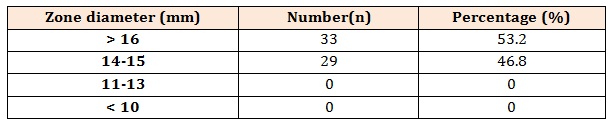
Table 2: Distribution of zone of inhibition for teicoplanin for 62 MRSA isolates.
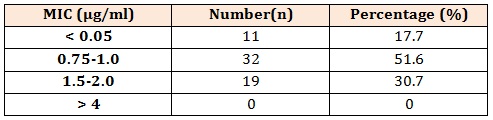
Table 3: Distribution of MIC values for teicoplanin for 62 MRSA isolates.