
Citation: Akeredolu DO and Akinnibosun FI. Isolation of Bacteria and Physicochemical Analyses of Petroleum-Products Contaminated Soil from NNPC/PPMC Depot, Benin City, Nigeria. J Microbiol Biotechnol, 2017, 2(2): 000118.
*Corresponding author: Akeredolu DO, Department of Microbiology, University of Benin, Benin City, Nigeria, Email: dennisakeredolu@gmail.com
This study was aimed to evaluate the physico-chemical properties and presence of bacteria of from soil samples contaminated with different petroleum products which are petrol (PMS), diesel (AGO), pure kerosene (DPK) and mixed kerosene (DPK) obtained from the tank farm of NNPC/PPMC Depot in Benin City, Nigeria. The mean count for heterotrophic bacterial ranged from 1.83x 104±0.09 cfu/g soil contaminated with mixed DPK to 5.93x104±0.02 cfu/g for soil contaminated with PMS. Nine (9) bacterial isolates were characterized and identified; Bacillus subtilis, Micrococcus varians, Pseudomonas aeruginosa, Klebsiellaaerogenes, Alcaligenes sp., Corynebacterium sp., Bacillus sp., Arthrobacter sp., and Pseudomonas sp.Bacillussp. and Pseudomonassp. were the most occurring bacteria isolates in the four soil samples. From the physico-chemical analysis, the pH value ranged from 6.50-6.70, electrical conductivity ranged from 14.20mhos/cm-32.50mhos/cm, moisture content from 10.20%-14.50%, carbon from 1.38%-2.97%, nitrogen from 2.37%-8.19%, 0.396%-0.950%, phosphate 1.95%-5.52% and total hydrocarbon from 125mg/kg-3,050mg/kg These results revealed that the presence of these bacteria under the stated soil condition can enhance bioremediation of petroleum contaminated soil. This study reveals the possible biodegradability of several petroleum products by bacteria which will aid the bioremediation of the contaminated soil.
Keywords: Bacteria; Physicochemical Analyses; Petroleum-Products; Contaminated Soil
Activities associated with petroleum exploration, development and production operations have local detrimental and significant impacts on the atmosphere, soils and sediments, surface and groundwater, marine environment, biological diversity and sustainability at terrestrial ecosystems in the Niger Delta [1]. Discharge of petroleum hydrocarbon and petroleum-derived waste, streams have caused environmental pollution, adverse human health effects, and detrimental impact on regional economy, socio-economy problem and degradation of host communities in the 9 oil-producing states in the Niger Delta region [1]. Recently, anthropogenic practices such as industrial activities, petroleum and petroleum derivatives (such as gasoline, diesel, and kerosene spills), and incomplete combustion of fossil fuels have caused an accumulation of petroleum hydrocarbons in the environment [2]. In fact, petroleum and derivatives have a major ecological impact on contaminated marine and terrestrial ecosystems [2]. Many important processes influence the destination of hydrocarbons in the environment. Among these are sorption, volatilization, abiotic transformation (chemical or photochemical), and biotransformation [3]. Biodegradation of oil contaminated soils, which exploits the ability of microorganisms to degrade and/or detoxify organic contamination, has been established as one of the efficient, economic, versatile and environmentally sound treatment [4].
The presence of a high enzymatic capacity allows microbial communities to degrade complex hydrocarbons [5]. This capacity to modify or decompose certain pollutants, such as petroleum, summarizes the importance of enzymes in the bioremediation process. Their genetic diversity contributes to the metabolic versatility of microorganisms for the transformation of contaminants into less-toxic final products, which are then integrated into natural biogeochemical cycles [5]. However, appropriate environmental factors such as pH, available nitrogen and phosphorus, Organic matter, moisture and carbon content are essential for the performance of these organisms. The application of nutrients to oil contaminated site to stimulate the growth of naturally occurring hydrocarbon utilizing bacteria for bioremediation purpose, can greatly improve the rate of recovering of environments contaminated with petroleum products. Therefore this study was aimed to Isolation of Bacteria and Physicochemical Analyses of PetroleumProducts Contaminated Soil from NNPC/PPMC Depot, Benin City, Nigeria.
Materials and MethodsSoil samples contaminated with petroleum products (diesel, petrol, pure and mixed kerosene) were collected from tank draining points from tank farm at NNPC/PPMC Benin deport. Samples were collected at different point from the tank farm into polyethylene bags which were duly labelled and transported to the laboratory. All glassware used were thoroughly washed, air dried and sterilized using an autoclave at 121oC for 15 min.
Physic-Chemical Analysis of Soil SamplesThe pH was determined electrometrically by suspending the soil in 1: 2 (soil: 0.01M CaCl2) mixture. The suspension of the soil was allowed to stand for 30 minutes with occasional stirring and the pH measured with a pH meter [6]. Moisture an aluminium dish was pre weighed (W1) using a sensitive weigh balance (State Model). Ten (10) grams of the fresh soil sample was transferred to the dish and weight of both the dish and soil was noted (W2). The dish containing the soil sample was placed in a hot air oven (State Model) at 1300C and dried to obtain a constant weight for 24 hours. The dish was immediately transferred to a desiccator and allowed to cool for 30 minutes. The resultant weight was taken (W3). The moisture content was calculated and recorded as a percentage by weight of the respective soil sample.
Total Organic carbonThe Total Organic Carbon was determined by the Walkley-Black titrimetric method. About 0.3 g of each soil samples were weighed into an Erlenmyer flask with 10 ml of K2CrO7 and 20 ml concentrationH2SO4 was added. The mixture was gently swirled until soil and reagents were properly mixed and were allow to stand for 30 min, 100 ml of distilled water was added and the content titrated against standardized ferrous sulphate solution to a reddish brown end point using ferroin as the indicator. The total organic matter was calculated from the value obtained for the total organic carbon [7].
Total hydrocarbon contentHydrocarbon-utilizing bacteria population was enumerated by spread plate technique [7]by inoculating 0.1 ml of aliquot onto sterile Mineral Salt Medium (MSM) plates with 100μl each of petroleum products viz; diesel (AGO), petrol (PMS), pure kerosene (DPK) and mixed kerosene (DPK). The petroleum products used was sterilized by filtering through Millipore filter,0.45μ diameter and stored in sterile bottles. The petroleum products were used as the sole carbon source to isolate hydrocarbon-utilizing bacteria. Theplates were incubated at 25° C for 3-5 days. Any isolate which grow on nutrient agar plates but failed to grow on mineral salt medium plate were confirmed as non-degraders. The isolates which grow on both the agar plates were confirmed as hydrocarbon utilizes. Total hydrocarbon content was calculated as described by[8] (Figure 1).
Available phosphorusOne gramme (1.0 g) of soil was shaken for 5 minutes with 10 ml of extracting solution containing 0.03 N NH4F and 0.1 N HCl). The solution was filtered through Whatman filter paper and 3 ml of the filtrate was transferred into a test tube and 3ml of ammonium molybdate was added. Thereafter, 5 drops of mixture of boric acid, sodium sulphite and sodium sulphates were added. The Phosphorus content was determined calorimetrically [9].
Available nitrogenOne gramme (1.0 g) of the soil sample was placed into Kjedahl digestion flask. One table of a catalyst and 20 ml concentrated tetraoxosulphate acid was added and the mixture was hand shaken to ensure mixing. At completion of digestion, 10 ml distilled water was added and the solution was filtered through a Whatman filter paper. Nitrogen was determined calorimetrically at 625 nm [9].
Total heterotrophic bacteriaTHB population was enumerated by pour plate method. 1g of the sample was aseptically transferred into 100ml of physiological saline and transferred in a series of eight 10 fold serial dilution using physiological saline. 1ml of the aliquot from each of the dilution of 102,104,106 and 108 was inoculated by pour plate method onto Nutrient agar (NA) in duplicates. The plates were incubated aerobically at room temperature for 48 – 72 hrs. The resulting colonies were counted and recorded as colony forming units per gram (cfu/g) of soil sample [10].
Characterization and Identification of IsolatesThe enumerated bacteria were isolated and stored in NA slants at 4°C for further identification. Primary identification was done on the basis of colony and cell morphology and Gram staining. Colonial examination of the isolates was carried out to determine the type of shape, elevation and pigmentation pattern they exhibited [11]. Secondary identification is carried out by performing a series of Biochemical tests,[12] (Table 1).
Results and DiscussionThe physicochemical parameters are show in Table 2. The pH of pure dual purpose kerosene (DPK) is slightly higher (6.7) than that of premium motor spirit(PMS), automobile oil and gas (AGO), and mixed dual purpose kerosene (DPK) which are 6.60, 6.50, and 6.60 respectively. The conductivity of pure DPK is higher (32.50mhos/cm) compared to the PMS, AGO and Mixed DPK. The moisture content of pure DPK was more with 14.50% while that of PMA AGO, and Mixed DPK were 10.20%, 10.80% and 10.20% respectively. Total Carbon content was higher in AGO (4.75%) compared to PMS, Mixed DPK and Pure DPK which have 2.19%, 1.38% and 2.97% respectively. Total organic matter, Nitrogen and Phosphate (8.19%, 0.95% and 5.52% respectively) were also higher in AGO. The total heterotrophic bacteria were higher in nutrient agar plates than in MSM which was spread with 0.1ml of petroleum products. The total heterotrophic bacteria count is shown in Table 3. The population density of heterotrophic bacteria in soil sample contaminated with PMS was 5.93×104±0.20 which was highest compared to the soil contaminated with AGO, pure DPK and mixed DPK with population densities of 3.53×104±0.15, 2.77x104±0.24 and 1.83x104±0.09 respectively.
KeyAGO - Automotive Gas & Oils
DPK - Dual Purpose Kerosene.
PMS - Premium Motor Spirit.Another name for gasoline.
The number of colonies on mineral salt medium is lower when compared to the mother plate without hydrocarbons. These results showed that the bacteria grown on enriched medium were able to utilize the hydrocarbon. The morphological and biochemical characterization of the bacterial isolates obtained from the different soil collected from different spot from the Tank farm revealed the following isolates; Bacillussubtilis, Micrococcusvarians, Pseudomonasaeruginosa, Klebsiellaaerogenes, Alcaligenes sp., Corynebacterium sp., Bacillussp., Arthrobactersp. and Pseudomonas sp. Bacillus subtilis, Pseudomonas aeroginosa, Bacillus sp., Pseudomonas sp. were present in all soil samples. Alcaligenes sp. was isolated from pure DPK, Micrococcussp. was present in PMS and Mixed DPK, Klebsiellaaerogenes present in Mixed DPK, Alcaligenessp. was present in AGO, Corynebacterium sp. Present in pure DPK, Arthrobacter sp. was present in pure DPK, while Pseudomonassp. was present in PMS. All the isolates are rod shaped except Micrococcus varians which is cocci in shape. Their cells are arranged singly except for the Bacillus sp. which occurs in chains. The biochemical test reveals all to be catalase positive, all negative for indole, citrate and lactose test, and all positive for glucose test with the exception of Arthrobactersp. Interestingly these organisms have been implicated in hydrocarbon degradation. Many scientists studied the petroleum degradation by various Pseudomonas species, Bacillus species, Micrococcussp and Alcaligenes [13-16]. Jyothi et al. [17] also isolated Bacillus species, Micrococcus luteus, Corynebacteriumxerosis from petroleum contaminated soil. The physico-chemical properties and microbial loads of soil showed that there are essential nutrients in soil especially nitrate and phosphate necessary for microbial growth. The microorganisms isolated were: Bacillussubtilis, Micrococcusvarians, Pseudomonasaeruginosa, Klebsiellaaerogenes, Alcaligenes sp., Corynebacterium sp., Bacillussp., Arthrobactersp., and Pseudomonassp. The presence of microorganisms in the hydrocarbon contaminated soil samples showed that soil indigenous microflora can metabolize crude oil or hydrocarbons in contaminated sites.
ConclusionThe presence of microorganisms in the hydrocarbon contaminated soil samples showed that soil indigenous microflora can metabolize crude oil or hydrocarbons in contaminated sites and these bacteria under the stated soil condition can enhance bioremediation of petroleum contaminated soil. This study reveals the possible biodegradability of several petroleum products by bacteria which will aid the bioremediation of the contaminated soil. Further research in genetically modified bacteria that will be able to degrade all types of petroleum products which will advance the bioremediation of contaminated soil.
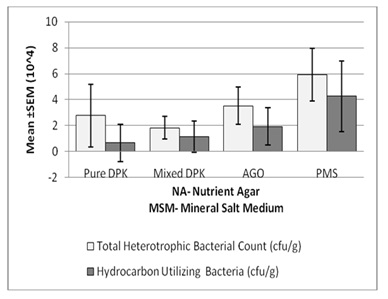
Figure 1: Total heterotrophic and hydrocarbon utilizing bacteria count (cfu/g)
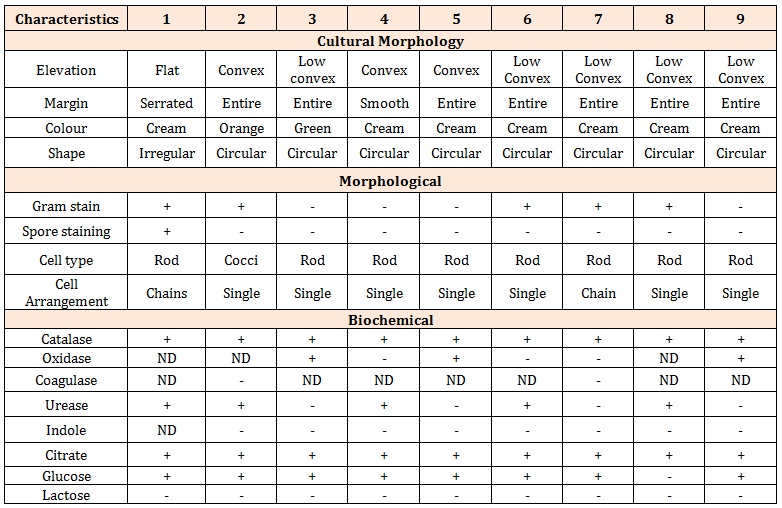
Table 1: Cultural, Morphological and Biochemical Characteristics of Bacteria Isolates Likely Identifiable organisms
*1= Bacillussubtilis *2= Micrococcusvarians *3= Pseudomonasaeruginosa *4= Klebsiellaaerogenes * 5= Alcaligenes sp. *6= Corynebacterium sp. *7= Bacillus sp.*8= Arthrobacter sp.*9= Pseudomonas sp.
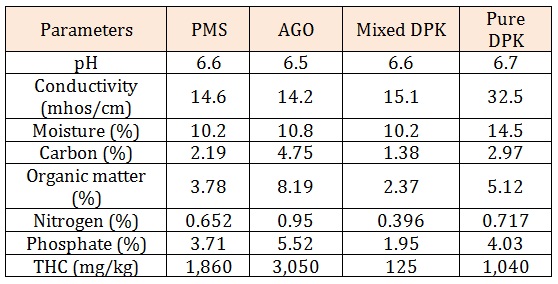
Table 2: Physicochemical parameters of the soil samples contaminated with petroleum products
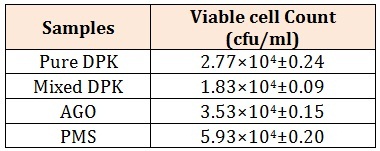
Table 3: Total Heterotrophic Bacterial Count (cfu/ml) Where DPK-dual purpose kerosene, AGO-automobile oil and gas and PMS- premium motor spirit