
Citation: Changxue Xu, et al. Biofabrication of Interpenetrating Polymer Network Hydrogels. Ergonomics Int J 2017, 1(3): 000114.
*Corresponding author: Changxue Xu, Department of Industrial, Manufacturing, and Systems Engineering, Texas Tech University, Lubbock, Texas, 79409, USA, Tel: 806-834-6014; Email: changxue.xu@ttu.edu
Interpenetrating polymer network (IPN) hydrogels are mixtures of multiple cross-linked polymers, which don’t have any covalent bonds between components, but cannot be separated using purely physical methods. The combination of the individual polymers results in a multi-component polymer system with a new profile with better mechanical properties and better swelling/deswelling response. This review paper aims to give an overview of the IPN hydrogels and the associated manufacturing techniques within a variety of biomedical applications. The first part of this review introduces different IPN-based hydrogels and their classification. The second part of this review focuses on different manufacturing techniques, which are used to process the IPN hydrogels to fabricate 3D constructs for various applications in biomedical engineering.
Keywords: Stereolithography; Proteoglycans; Polyacrylamide; Microextrusion; Micromolded
The unavailability of organs for transplantation has resulted in a more and more severe organ shortage crisis [1]. For example, For example, U.S. Department of Health & Human Services reports that more than 120,000 people are on the waiting list for different organ transplants in U.S. alone, and it is estimated that a new patient is added to the national organ transplant waiting list every 10 minutes [2]. Fortunately, organ printing, which is defined as a computer-aided, layerby-layer additive biofabrication of three-dimensional (3D) functional human tissue and organ constructs using tissue spheroids as building blocks [3], is emerging as a novel and promising solution to solve the organ donor shortage crisis. The fabricated tissues and organs are envisioned to be suitable for regeneration, repair and replacement of damaged, injured or lost human organs [4,5]. The interaction between the living cells and the microenvironment (in particular the extracellular matrix) is very critical for successful tissue/organ printing. The extracellular matrix is a dynamic ensemble of proteins and proteoglycans, which surrounds cells, provides anchoring sites, and regulates growth of factor signaling [6]. Hydrogels have been widely used to mimic the natural extracellular matrix in our body.
Hydrogels are crosslinked polymers, which exhibit the ability of swell in water and retain large amounts of water within their three-dimensional networks, but will not dissolve in water [7]. The main properties of hydrogels make them advantageous for use in a variety of biomedical applications including the high content of water, biocompatibility, and biodegradability [8], which are analogous to those of the natural extracellular matrix [9]. Moreover, their soft nature minimizes irritation to surrounding tissues, and they can be modified with ligands to improve cell adhesion. Typically, hydrogels are produced by crosslinking a hydrophilic network in a specific manner. The crosslinking mechanism can be classified into two categories: physical crosslinking and chemical crosslinking. Different crosslinking mechanisms affect the manufacturing processes, and the mechanical properties. Physically crosslinked hydrogels have temporary bonds, such as ionic bonds, hydrogen bonds, hydrophobic forces, and polymer chain entanglements [10], and they are mostly reversible. Ionic hydrogels are widely used physical crosslinked hydrogels due to excellent cytocompatibility, such as crosslinking of alginate by the addition of calcium ions [11]. Thermal crosslinking is triggered by interaction between polymer chains in the solution due to temperature change, such as crosslinking of gelatin by cooling, which results in the conformational transition of gelatin chains from coil to triple helices [12]. Chemical crosslinking yields hydrogels held together by covalent bonds between monomeric chains. These hydrogels are permanent and have higher mechanical strength compared to their physically crosslinked counterparts [13]. Free radical polymerization is one of the most commonly used techniques in 3D bioprinting to form chemically crosslinked hydrogels. Polymers with vinyl groups are exposed to UV irradiation, which induces the formation of free radicals to combine the polymer chains together into 3D networks through covalent bonds [14]. Some examples of hydrogels that undergo crosslinking in this manner include polyethylene glycol (PEG) [15], polyvinyl alcohol (PVA) [16], and polyacrylic acid (PAA) [17]. Chemical crosslinking can also be achieved by addition reactions, in which functional crosslinking agents can react with polymers via addition reactions, such as the reaction of PEG-dithiol with PEG-acrylates to produce a degradable hydrogel [18].
Despite of their widespread use in biomedical applications, hydrogels have been recognized to possess inherently weak mechanical properties, requiring the need for additional measures to produce robust constructs [19,20]. Recent advances in the interpenetrating polymer networks (IPNs) have enabled hydrogels with better mechanical properties and better swelling/deswelling response. This review paper will focus on the IPN-based hydrogels and their associated biofabrication technologies.
IPN Hydrogels and ClassificationIPNs can be described as mixtures of multiple crosslinked polymers, without any covalent bonds between them, but cannot be separated using purely physical methods [21]. Essentially, the combination of the individual polymers results in a multicomponent polymer system with a new profile [22].
IPNs can be classified according to the preparation methods shown in Figure 1: simultaneous IPNs and sequential IPNs. In simultaneous IPNs, the precursors of the individual components are mixed and the individual networks are crosslinked simultaneously by noninterfering routes of polymerization [23,24], such as an IPN hydrogel composed of polyacrylamide (PAAm) and Pluronic, where crosslinking of the precursor solution mixture was achieved using a redox initiator resulting in the simultaneous crosslinking of both Pluronic and PAAm [25]. In sequential IPNs, the mixture of the individual components is subject to the first crosslinking resulting in a single-network hydrogel containing a solution of the other monomers, and then the other monomers are crosslinked to form IPNs that have the second network embedded within the first network [23,26], such as a PEG-collagen IPN hydrogel, which was prepared by mixing collagen and poly(ethylene glycol) diacrylate (PEGDA) precursor solutions, and crosslinking the PEG under a UV light followed by physical crosslinking of collagen by incubation at 37°C [27].
IPNs can also be classified based on the precursor solutions used in preparing the IPNs: natural polymers and their derivatives, and synthetic polymers [21]. Common natural polymers used in biomedical applications include polysaccharides (such as alginate and chitosan) and proteins (such as gelatin, collagen and silk fibroin) [28]. For example, gelatin and alginate were used as precursors to prepare gelatin-alginate IPN hydrogels using a combination of enzymatic and ionic crosslinking approaches [29]. Alginate and fibrin were used as precursors to form a dynamic IPN hydrogel that was used for the in vitro growth of ovarian follicles [30]. Collagen and chitosan were mixed to prepare a collagen-chitosan IPN hydrogel for the in vitro culture of human epidermoid carcinoma cells. Methacrylated gelatin and silk fibroin were used as precursors to synthesize an IPN hydrogel with robust mechanical properties for applications in encapsulation of NIH-3T3 fibroblasts [31]. Chitosan and silk fibroin were used to synthesize a tough IPN hydrogel that could be utilized as artificial muscles [32]. Common synthetic polymers used as precursors include polyacrylamide (PAAm) [33], polyethylene glycol (PEG) [34], polyvinyl alcohol (PVA) [35], polyacrylonitrile (PAN) [36], to name a few. For example, PEG and PAAm were used as precursors to synthesize an IPN hydrogel for the purpose of enzyme immobilization [37]. PVA and PAAm were used as precursors to prepare an IPN hydrogel that exhibited significantly improved mechanical properties over the individual components [38]. IPN hydrogels used in biomedical applications can also be generated by combining the natural and synthetic hydrogels together as precursors. For example, alginate and PVA were used to prepare an IPN hydrogel that supported the growth and survival of fetal cardiac cells for applications in cardiac tissue engineering.
Chitosan and PVA were used to prepare a biodegradable and mechanically robust IPN hydrogel [39]. Chitosan was used as a precursor along with polyacryloglycine to synthesize a pH sensitive and thermally responsive IPN hydrogel for drug release applications [40]. PEG and fibrinogen were utilized to synthesize an IPN hydrogel that was used as scaffolds for 3D cell cultures [41].
Manufacturing Techniques for IPN HydrogelsDifferent manufacturing techniques have been employed for the biofabrication of IPN-based 3D constructs for a variety of biomedical applications, such as molding, stereolithography, microextrusion, to name a few.
In molding, the precursor solutions are injected into a mold, and subject to a primary crosslinking process to enable crosslinking of one constituent network, followed by subsequent crosslinking after removal of the construct from the mold. Brigham, et al. [42] utilized molding to fabricate an IPN-based scaffold for NIH-3T3 cell seeding using methacrylated hyaluronic acid (MeHA) and collagen shown in Figure 2a. It was reported that compared to individual MeHA and collagen, the IPN hydrogel had improved cell adhesion on the surface and better mechanical properties. Collagen and glycidyl methacrylate modified hyaluronic acid (GMHA) precursors were put in a silicone mold, and subject to selective photo crosslinking under a UV lamp to fabricate IPN-based patterned scaffolds, which is demonstrated to be very effective for seeding cells [43]. Biophysical characterization performed on the IPN hydrogels indicated that they were mechanically strong and included a highly interconnected porous network structure, which rendered them effective as scaffolds for tissue engineering compared to pure GMHA structures, which have been observed to be resistant to cell attachment and spreading. Moreover, it was observed that the mechanical properties of the IPN constructs could be easily tailored by changing the ratio of the precursors [44]. Molding is a traditional manufacturing technique with advantages including accurate control over small features and reusable molds. However, a big limitation for molding is fabrication of heterogeneous and complex constructs [45].
In stereolithography, the photopolymer exposed to a light source (such as a UV lamp) to solidify the pattern layer by layer to generate 3D constructs. A knee meniscus structure was fabricated using stereolithography with an IPN hydrogel composed of PEGDA and collagen shown in Figure 2b [46]. Mechanical testing on the construct revealed a greater shear modulus than the constructs made with one of the constituents alone. It was reported that the properties of the IPN could be modulated by changing the concentration of the constituents, and typical problems with stereolithography on hydrogels, namely high shrinkage and curling, were not observed. Li, et al. [47] utilized stereolithography to fabricate dogbone-shaped structures composed of a simultaneous IPN hydrogels with acrylate and epoxy as the constituents by manipulating the formation of the cationic network. Graded IPN constructs with varying mechanical properties were successfully printed by varying curing times along the construct, which resulted in a variation in mechanical properties on the order of millimeters.
In microextrusion, the precursor solutions are extruded out from a dispensing head to form filaments, which are crosslinked to create 3D constructs layer by layer. Wei, et al. [48] fabricated 3D meniscus-shaped structures using agar, alginate and PAAm as constituent components. The addition of alginate not only increased the ink viscosity for precise processing control, but also restricted the agar helical chain bundles from pulling out under stress to toughen the IPN hydrogels. Nanocellulose and alginate were extruded to fabricate 3D complex anatomically shaped constructs, such as ear and sheet meniscus constructs [49]. Human nasoseptal chrondocytes were encapsulated in these structures with high cell viability.
The new advance in the microextrusion-based printing of IPN hydrogels is to introduce a microgel bath to assist the micro extrusion process for fabrication of 3D vascular-like constructs of PEG-alginate IPN hydrogels shown in Figure 2c [50]. During the fabrication process, the microgel bath is used as a substrate to support and maintain the shape of the fabricated 3D structures, although the fluid printed is not crosslinked. Then, the fabricated structures are subject to a two-step gelation process to successfully form 3D vascular-like constructs of IPN hydrogels.
ConclusionsIPN hydrogels have gained more and more attention in recent times due to their superior mechanical properties. The advances in 3D biofabrication techniques have enabled the creation of anatomically shaped IPN hydrogel-based 3D constructs for a variety of applications in biomedical engineering.
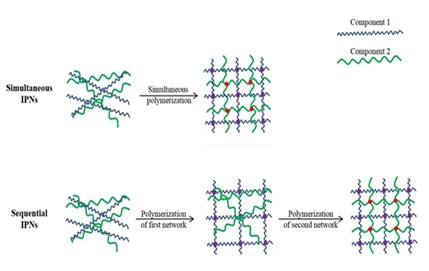
Figure 1: Schematics of simultaneous IPNs and sequential IPNs.
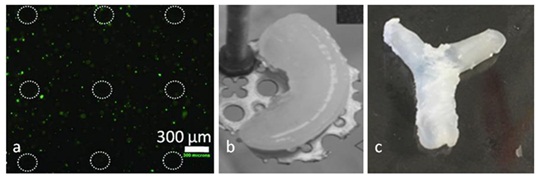
Figure 2: Various IPN-based 3D constructs fabricated using different manufacturing techniques:
a) Micromolded scaffold made of MeHA-collagen IPN hydrogels [42]
b) Knee meniscus structure fabricated using stereolithography [46]
c) Bifurcated tubular structure fabricated using microextrusion