
Citation: Imai J, et al. The Specialized Sub Cellular Compartments for Cross-Presentation in Dendritic Cells. J Pharm Res 2017, 1(3): 000121.
*Corresponding author: Jun Imai, Laboratory of Physiological Chemistry, Faculty of Pharmacy, Takasaki University of Health and Welfare, Takasaki, Gunma 370-0033, Japan, Tel: +81-27-352-1180 (8326); Email: jimai@takasaki-u.ac.jp
Major histocompatibility complex class I (MHC I) molecules are expressed on the surface of all nucleated cells associated with short antigenic peptides about ten amino acids long at their extracellular part. The antigenic peptides are derived from endogenous proteins, which are processed by the ubiquitin-proteasome system in the cytosol. In contrast, exogenous proteins are processed by lysosomal proteases and resultant antigenic peptides are presented upon Major histocompatibility complex class II (MHC II) molecules. In several kinds of cells, especially in dendritic cells (DCs), exogenous proteins are processed and presented upon MHC I in addition to MHC II. This phenomenon is called crosspresentation (CP). CP plays important roles not only in activations of CD8+ T cells into cytotoxic T lymphocytes (CTLs) against anti-infectious and anti-tumor immunity but also in in-activations of self-acting CD8+ T cells by T cell energy or T cells deletion for peripheral tolerance. To this date, because of the important roles for CP, there are accumulating evidence to elucidate the molecular mechanisms of CP. Recent researches indicated that the endoplasmic reticulum (ER)- associated degradation (ERAD) pathway plays a central role in processing of exogenous proteins. The conveyances of exogenous proteins into ERAD-possible compartments are dependent upon the integration among endosomal or phagosomal membrane with ER membrane. But there still remain several important questions about CP, such as the precious molecular mechanisms of these integrations and the peptide-loading mechanisms upon MHC I. This review describes the recent researches about the molecular mechanisms of CP.
Keywords: Dendritic cells; Cross presentation; Major histocompatibility complex class I; Endoplasmic reticulum; Peptide loading complex
Major histocompatibility complex class I (MHC I) corresponds to the human leukocyte antigen (HLA) -A, -B, and -C are found on the surface of all nucleated cells [1]. However these HLAs are famous for the major cause of organ transplant rejections, they play important roles in disease defense against in-cell parasite bacteria, viruses, and cancers. MHC I bound with antigenic-peptides about ten amino acids long at their extracellular part and displays these antigenic-peptides against CTLs [1]. These antigenic peptides are generated from endogenous proteins by the ubiquitin-proteasome systems. CTLs specifically recognize these MHC I molecules with non-self antigenic peptides by their surface molecular complex called T cell receptor (TcR) and differentiate self-cells and non-self cells. After ligation of TcR with MHC I with nonself antigenic peptides, CTLs release the cytotoxins (perforin, granzymes, and granulysin) [2] or express the FAS ligand (CD95L) on their surface [3]. Either process eventually leads apoptosis against those non-self cells. In distinction from MHC I, expression of MHC class II (MHC II) is restricted to only professional Antigen-presenting cells (without mentioning described as APC) [4]. APCs have specific abilities to show antigenic peptide from extracellular proteins upon MHC II against T cells. MHC II interacts with TcR of CD4+ T cells and regulates these CD4+ T cells to start appropriate immune responses [4].
MHC I molecules shows high polymorphism and most of their variations are concentrated around peptide binding groove at their extracellular part [1]. This diversity enables MHC I to show a wide variety of antigenic-peptide against immune systems and enables our immune system to react with various diseases. To insure the multiplicity of antigenic peptide, our immune system furnished with splendid molecular apparatus to supply the diversity of antigenic peptide upon MHC I. Antigenic peptides are processed by the ubiquitinproteasome system in the cytosol from endogenous proteins [1] and then are transported into ER trough the transporter associated with antigen processing 1 (TAP1)/TAP2 complex (TAP complex) [5,6]. In ER, antigenic-peptides are inserted into peptide binding groove of newly translated MHC I with the aid of the peptide-loading complex (PLC), which is consistent from Tapasin, Calreticulin, and ERp57 (PDI) other than TAP complex. TAP complex transports a wide variety of peptides into ER, because of their low specificity against substrates [5,6]. In ER, MHC I are connected with TAP complex by Tapasin so as to guarantee the rapid loading of an antigenic peptide. These in no time responses also protect these antigenic peptides from high amino peptidase activity in both cytosol and ER. In addition, these peptide loadings are assisted by specific chaperones, Calreticulin and ERp57 (PDI), which ensure the correct folding of MHC I complex. After binding with an antigenic peptide, the MHC I is released from the PLC and are transported toward the cell surface. These subsequent processes are called direct-presentation (DP) [7].
Cross-PresentationCTLs, which destroy cancer cells or infected cells, are derived from CD8+ T lymphocytes. However the number of each antigen-specific CD8+ T cells is too small, and in addition, they cannot destroy non-self cells before differentiation into CTLs. To revitalize non-activated CD8+ T cells into CTLs and to proliferate them into enough numbers, two stimulations are indispensable; specific TcR ligations against MHC I complexes loaded with antigenic peptide and CD28 against co-stimulatory molecules (CD80, CD86) upon APCs [8,9]. Among these two stimulations, the ligation between TcR and MHC I complex determines the specificity of CTL. Since CTLs can only destroy target cells with just the according to antigenic peptides upon MHC I as APCs, APCs would present antigenic peptides derived from specific proteins of infected cells or cancer cells [10]. For this purpose, APCs have a specific ability to take up and process extracellular proteins so as to present those resultant non-self peptides upon their own surface MHC I. These sequential processes are called antigen cross-presentation (CP) and play definitive roles in the decision of the target cells for CTLs [10-13].
In contrast to TcR-MHC I signal, the co-stimulatory signal decides the general direction of differentiation upon CD8+ T cells. Co-stimulatory molecules are expressed only upon activated APCs by innate immunity. When CD8+ T cells are activated by TcR-MHC I signal together with the co-stimulatory signal, CD8+ T cells differentiated into CTLs. These activations are called cross-priming [14]. To the contrary, if CD8+ T cells are activated by TcR-MHC I signal by steady state APC without the co-stimulatory signal, CD8+ T cells fall into energy or are led into apoptosis. Those in activations are designated as cross-tolerance [14-17].
Dendritic CellsAPCs include macrophages, B cells, and dendritic cells. They show specialized ability in presenting antigen to T cells. They also express a set of pattern recognition molecules for discrimination of kinds of non-self in their environments to decide the activations and direction of acquired immunity. Among above three APCs, DCs show the strongest ability of CPs. The name of DC is derived from their arboroid branch structure (dendrite) found in draining lymph node. DCs, kinds of hematopoietic bone marrow progenitor cells, found in the almost whole of our body. Immature DCs continuously explore the surrounding environment by pattern recognition molecules and incorporate exogenous proteins. After migration into draining lymph node, DCs project many branches to interact with T cells and B cells trough their surface MHC complex to initiate and shape the adaptive immune response, which is dependents upon the information from their pattern recognition molecules. But for the indispensable roles of DCs in the adaptive immunity, they are heterogeneous series of subsets derived from several precursors. DCs are further divided into three main populations by steady-state phenotypes: plasmacytoid DCs (pDCs), conventional DCs (cDCs), and monocyte-derived DC (moDC also known as inflammatory DC) [18,19]. These three populations are further divided into detailed subsets by their surface antigens. Among these multiple DCs, cDC (CD8+ CD11b- subset and CD103+ CD11b- subset) and mo-DCs shows highest CP efficiency [20,21].
ERAD Utilized Processing of Extracellular Proteins in CPPrevious studies about CPs found out two major pathways for transports and processing of extracellular proteins; one is the proteasome pathway and another is the endosome/lysosome pathway. The former pathway is also known as the TAP-dependent pathway and the latter pathway is also described as the TAP-independent pathway. But recent researches about graft rejection have shown that the TAP-dependent proteasome pathway is the principal pathway in CP [22].
However in the proteasome pathway, exogenous proteins are transported into the cytosol so as to be processed by the ubiquitin proteasome system, they are not incorporated into the cytosol directly. Exogenous proteins are incorporated into endocytic compartments, such as phagosome or endosome at first. In CPs, endogenous proteins are transported through the lipid bilayer before degradation by the lysosomal protease. This particular activity to transport whole exogenous proteins through lipid bilayerinto the cytosol is eminent in DCs. Other APCs such as macrophages and B cells are below DCs in this specific ability [23]. To this end, DCs are furnished with two appropriate abilities; one is a low proteolytic ability of lysosomal proteases and the other is the high efficiency to transport exogenous proteins into the cytosol. To protect exogenous proteins from lysosomal proteases, DCs shows the low activity of lysosomal proteases [24] by keeping phagosome and endosome/lysosome under alkaline pH conditions as 7.5- 8 [25,26]. In addition, inhibition of lysosomal proteases results in acceleration of CP efficiency in human moDCs or cDC [27-29]. To pass exogenous proteins through cellular membrane, DCs utilize the molecular apparatus of ERAD.ERAD is a universal cellular pathway, which identifies misfolded proteins in ER, transports them into the cytosol trough ER membrane, and finally degrades them by the ubiquitin-proteasome system[30-32]. Inhibition of some of these ERAD-related molecule impaired CP ability strongly indicated the essential roles of ERAD in CP [33-35]. But for those important roles of ERAD in CP, recent research indicated that the transport of exogenous proteins into the cytosol is carried out in endocytic compartments and not in ER. ERAD-related molecules are localized to phagosome or endosome [36- 38]. In addition, a restricted localization of the Sec61 complex, which is the singular translocon into CP, in ER strongly, inhibited CP [39]. Those results indicate that transports of exogenous proteins out of lipid bilayer was carried out neither in ER nor in the classical endosome, but in endosome with ER resident molecules [39].
A Cellular Compartment for Peptides Loading upon MHC IThe cellular compartments for the peptide loading on MHC I in CPs are still controversial. Strong amino peptidase activities both in the cytosol and in ER suggest that antigenic peptides are inserted into peptide binding groove soon after processed by ubiquitin proteasome system. In addition, the variations of antigenic peptides upon MHC I is significant for acquired immunity, the peptide loading is carried out with the aid of PLC as DP. These presumptive evidence give us suggestions that the peptide loading in CPs is carried out in above endosome with ER resident molecules. Recent researches support the hypothesis. (i) Observations by optical microscopes and electron microscopes showed that PLC molecules were also localized in endosome and phagosome [34,36- 38,40,41]. (ii) The transportation of MHC I into endocytic compartments was essential for CP [42-44]. (iii) Fully conformed MHC I without antigenic peptide was found in endocytic compartments [45]. (v)CD74 showed an important role in CP by regulating the transport of MHC I into endocytic compartments in DCs [43]. Of late, we purified the sub cellular compartment where exogenous proteins undergo ERAD processing from moDCs derived cell line DC2.4 [46]. In our purified microsome we could detect both ER-resident proteins and endocytic compartment-specific proteins; ER-resident proteins (Sec61-α and GP96), MHC I and PLC proteins (Calreticulin, ERp57 (PDI), Tapasin, and TAP complex), ERAD-related proteins (CHIP, VCP, and proteasomes), and endosomespecific proteins (Lamp1 and Rab5). Among these molecules, it is note worthy that Lamp1 was detected as both in the precursor form and in the mature form and that fully conformed MHC I were loaded with the pOVA epitope (Figure 1). In addition using our purified compartment, we reconstituted processing of extracellular proteins in vitro [46]. Those results strongly suggest that these purified microsomes correspond to the non-classical endocytic compartment for CPs, which is responsible for both processing of exogenous proteins and peptide loading upon MHC I. We are now attempting in vitro reconstruction of peptide loading using our purified microsome.
Molecular Mechanisms of Integrations among ER and Endocytic CompartmentsBut for the amassed efforts to elucidate the molecular mechanisms of CP, the precious molecular mechanism for the localizations of ER-resident proteins into endocytic compartments remained to be clarified. Recent researches shed a light on this question. (i) SEC22b played a significant role by regulating the fusion of phagosome and ER-Golgi intermediate complex [47]. (ii) Transport of MHC I into endocytic compartments are regulated by Rab22a [48]. (iii) Rab43 is essential in cDCs but not in moDC [49]. (iv) p38α plays important roles both in CP and cytokines production in cDC [50]. (v) CP efficiency is enhanced by activation of innate immunity [51]. All these results strongly suggest that DCs flexibly integrate membrane transport pathway by responding to environmental conditions for CP.
Concluding RemarksBut for the important roles of CP in both activation and inactivation of cell mediated immunity, molecular mechanisms for CP are not fully clarified yet. Accumulating findings strongly suggest that CP is dependent upon the inimitable vesicle transport systems in DCs. So as to integrate innate immunity and adaptive immunity, DCs acquire environmental information by their pattern recognition molecules and shape the most efficient immuno response. DCs equipped several redundant pathways for antigen presentations, which is running alongside with each other, and change vesicle transport system flexibly to fit environmental situations. To apply CP for DC-based immuno therapy, the elucidations of molecular mechanisms of CP are the issues of importance in researches of immunology field.
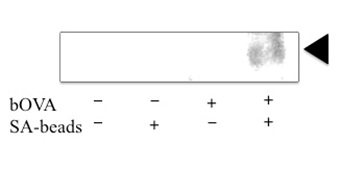
Figure 1: Microsomes with (+) or without (-) prior addition of bOVA were purified with (+) or without (-) SAmagnetic beads as described in our previous article [46]. Proteins (10μg) or corresponding volumes of purified proteins were immuno precipitated by 25D1.16 antibody which specifically recognize H2Kb allele of MHC I loaded with pOV8 epitope. After SDS-PAGE blotting was performed with anti-H2Kb antibody. A triangle in right side indicates the H2Kb.