
Citation: Molinos E, et al. To Comparison of Standard and New Chelating Solutions in Endodontics. J Dental Sci 2017, 2(3): 000131.
*Corresponding author: Molinos E, Department of Endodontics, School of Dentistry, University of Barcelona, Spain, Fax: +34 934 035 558, Tel: +34 934 024 268, Email: emolinsa@hotmail.com
Background: The use of 17% EDTA, followed by NaOCl irrigation during root canal instrumentation has been reported to effectively remove the smear layer from the root dentine walls. But EDTA is considered a pollutant and so it would be useful to identify alternative agents that offer greater biocompatibility.
Materials and methods: The root canals of fifty single-rooted were randomly divided into five groups (n=10): 0.3% chitosan, 17% EDTA, 20% citric acid, 2.25% peracetic acid, and a control group (deionized water). The total volume of each chelating solution was collected and analyzed by FAAS for quantification of calcium ions. The roots were split longitudinally and examined by SEM for assessment of smear layer removal. Results underwent statistical analysis by one-way analysis of variance (ANOVA) and Tamhane’s test.
Results: 17% EDTA and 20% citric acid showed similar capacities for smear layer removal, with significant difference in comparison with 0.3% chitosan and the control groups; there were no significant differences between different parts of the roots. The highest calcium ion concentration was observed with 20% citric acid.
Conclusion: 20% citric acid, 17% EDTA, 2.25% peracetic acid and 0.3% chitosan all removed the smear layer from the middle and apical thirds of root canals.
Keywords: :Chelating agents; Chitosan; Citric acid; Irrigation; Smear layer
Abbreviations: EDTA: Ethylene Diamenine Tetraacetic Acid; SEM: Scanning Electron Microscopy; FAAS: Flame Atomic Absorption Spectrophotometry
Irrigation is a key part of root canal treatment, essential for the removal of microorganisms, necrotic tissue, and the smear layer. The root canal’s smear layer stimulates the adhesion of microorganisms and also compromises the action of disinfectant solutions. Total elimination of the smear layer will improve the adaptation of filling materials, reduce apical and coronal microleakage of the root filling materials, and facilitate the diffusion of the irrigant to the root canal [1]. The most extensively used irrigant in root canal treatment is sodium hypochlorite (NaOCl) at concentrations of 1%- 5.25%. Research has shown that there is no difference in the antibacterial activity between 1%, 2.5% and 5.25% concentrations [2] but there is evidence that 4% sodium hypochlorite is more effective than saline solution (control) against Enterococcus faecalis in disinfecting root canals [3]. NaOCl’s antimicrobial activity and capacity to dissolve organic tissues is clear but its limited capacity to remove the smear layer from root dentine constitutes a major disadvantage. In order to decalcify dentine, chelating agents react with the calcium ions of the tooth structure, dissolving the inorganic structure of dentin by their low pH [4]. Ethylene diamenine tetraacetic acid (EDTA) is the most commonly used irrigant for smear layer removal. EDTA reacts with calcium ions in dentine, resulting in decalcification of the dentine within 5 min at approximate depths of 20-30µm [5].
The use of 17% EDTA, followed by NaOCl irrigation during root canal instrumentation has been reported to be effective in removing the smear layer from the root dentine walls [6]. But EDTA is a substance not found in nature and is considered a pollutant [7]. For this reason, the search for chelating solutions those are more biocompatible than EDTA has been documented with increasing frequency in the literature. Citric acid is a chelating agent that reacts with metals to form a nonionic soluble chelate. Citric acid has similar effects to EDTA in terms of smear layer removal [8]. It reacts rapidly with calcium ions and is less cytotoxic to tissues than EDTA [9]. Chitosan is a natural polysaccharide which is obtained from the acetylation of chitin, derived from the shells of prawns and crabs [10]. It has properties of biocompatibility, biodegradability, bioadhesion and is not toxic to the human body [11]. It has an acidic pH, shows a chelating capacity for different metal ions, and is used in various sectors of industry. Chitin is one of the most abundant substances in nature, which makes its use ecologically and economically attractive [10]. Peracetic acid is an ideal antimicrobial agent because of its high oxidation potential. It is widely effective against microorganisms, with sporicidal, bactericidal, virucidal and fungicidal actions at low concentrations [1]. The compound removes microorganisms by oxidation and subsequent rupture of the cell membrane by the hydroxyl radical. It decomposes to safe and environmentally friendly by-products, such as acetic acid and hydrogen peroxide. The aim of this study was to evaluate the efficacy of different chelating solutions for smear layer removal using scanning electron microscopy (SEM), and to quantify the concentration of calcium ions left in these chelating solutions after root canal irrigation using flame atomic absorption spectrophotometry (FAAS).
Materials and MethodsThe study design was approved by the Ethics Committee for Clinical Research at the Dental Hospital, University of Barcelona, Spain (code: EUDRACT number 2014-14). Fifty single-rooted human teeth were removed from storage in distilled water at 5º C. The crowns were shortened to a full root length of 12 mm measured from the apex using a laboratory hand piece and a diamondcoated microsaw blade (Osung USA, Pearland, USA). A size 10-K file (Dentsply Maillefer, Ballaigues, Switzerland) was introduced passively into each canal until its tip was just visible at the apex, to confirm apical patency. The working length (WL) was established as 12 mm. The root canals were prepared to an apical size of 30 (F3) with ProTaper Universal rotary instruments (Dentsply) driven by an X-Smart Plus (Dentsply) electric motor. Throughout preparation, the canals were irrigated with 2 ml of 2.5% NaOCl at each change of instrument, to give a total volume of approximately 10 ml. A 10-K file was used to maintain patency between each instrument.
Distribution for Final IrrigationThe teeth were randomly divided into five groups of equal size (n=10), according to the final irrigation solution used for smear layer removal: 0.3% chitosan, 20% citric acid, 2.25% peracetic acid, 17% EDTA, and a control group (deionized water). The chelating solutions used in this study were provided by the Institutional Endodontic Research Laboratory (Barcelona, Spain). In each group, all teeth were placed in a 50 ml sterile container, and the lid replaced by a perforated silicone stopper. In this way, the tooth could be positioned with the crown outside the container and the root inside. Afterwards, 5ml of the respective chelating solution were injected into the root canal for 2 min, using a 0.3 x 40 mm needle (Miraject Endo Luer, Hager & Werken, Duisburg, Germany), passing through the entire root canal, and exiting through the foramen into the collection container. Immediately after irrigant application, all samples were flushed with 3ml deionized water. The total volume of solution collected in the containers was later used for quantification of calcium ion concentration by FAAS.
FAAS AnalysisAfter collecting a total of 20 ml solution per specimen, the containers were lidded, labelled, and sent on for spectrometric determination of calcium ion concentration within the liquid. Calcium content was evaluated using an atomic absorption spectrophotometer (Perkin Elmer Optima3200RL, Waltham, Massachusetts, USA) with an air-acetylene flame. The dilutions were performed with 1% nitric acid (HNO3) at the following respective proportions: 1/10 for the 0.3% chitosan, 20% citric acid and 2.25% peracetic acid groups; 1/50 for the 17% EDTA group. These dilutions were necessary for the correct measurement of the concentration of calcium ions in solutions. Pattern solutions (Inorganic Ventures, Christiansburg, Virginia, USA) were used.
SEM AnalysisTwo diametrically opposed grooves were made in the teeth using metallic discs under water cooling. The hemisected piece with fewer irregularities representing the best total root canal length was selected. Only two specimens per group underwent observation. Each selected specimen was measured lengthways for delimitation of three equal third parts marked from the apex: apical, middle and coronal. Each area was analyzed by a single observer in high resolution images (4000x) captured with a scanning electron microscope (Model Quanta 200, FEI, Hillsboro, Oregon, USA).
The observer was trained to assess the amount of smear layer remaining on the dentine walls giving a score from 1 to 5 according to the following scoring system modified from Takeda [12] 1 point, smear layer covering the entire surface; 2 points, smear layer partially covering the surface and few visible tubules; 3 points, about half the surface with smear layer and half with open tubules; 4 points, smear layer covering a small amount of surface and visible tubules; 5 points, absence of smear layer on the surface.
Statistical AnalysisData pertaining to calcium eluted from root canals (ppm) were checked for normality and homoscedasticity among groups using quantile plots and Levene’s test. Comparisons between groups were made using one-way analysis of variance (ANOVA). When significance was detected and in response to the heterogeneity of variances, pair wise comparisons between groups were made applying Tamhane’s test.
ResultsTable 1 shows descriptive statistics for calcium ion concentration in each chelating solution. Calcium concentrations showed significant differences between different groups (Welch F4,19.02=103.4, p<0.001). The highest mean calcium ion concentration was observed in 20% citric acid (54.51 ppm) and 17% EDTA (52.69 ppm), followed by 2.25% peracetic acid (43.45 ppm) and 0.3% chitosan (33.78 ppm). Tamhane’s test found no significant differences between 20% citric acid, 17% EDTA and 2.25% peracetic acid. Calcium ion concentration in 0.3% Chitosan was significantly lower than 20% citric acid and 17% EDTA and the control group was significantly lower than the other experimental groups. Table 2 shows the smear layer scores in the coronal, middle and apical thirds of the root canals in each experimental group. SEM analysis revealed similar results between 0.3% chitosan, 20% citric acid, 2.25% peracetic acid and 17% EDTA which indicated only slight smear layer remnants on dentine walls, with significant differences between the experimental groups and the control group (Figures 1-3).
DiscussionThis study used scanning electron microscopy (SEM) to assess smear layer remnants on dentine walls and flame atomic absorption spectrophotometry (FAAS) to quantify the concentration of calcium ions in the chelating solutions assayed [10]. The chelating solutions were used for final irrigation following root canal instrumentation. SEM analysis found that 20% citric, 17% EDTA, 0.3% chitosan and 2.25% peracetic acid produced similar degrees of smear layer removal, while FAAS evaluation found that 20% citric acid, 17% EDTA and 2.25% peracetic acid obtained higher concentrations of calcium ions than 0.3% chitosan. The combined use of FAAS and SEM to assess the efficacy of chelating agents was based on previous studies [7,10]. Nevertheless, an ideal experimental model should have a longitudinal observational character, in which a given dentin area can be observed at different times. In this sense there is a need to improve experimental models for assessing smear layer removal in order to optimize the clinical guidelines for the chemical treatment of root dentin [13]. The results of the study are in agreement with other studies in that NaOCl alone does not effectively remove the smear layer from the dentinal walls because of its low physicochemical action that only works on organic particles [14]. But NaOCl coupled with EDTA can remove the smear layer formed in the instrumented root canals, mainly in the middle and coronal thirds [15]. There is no agreement as to an optimum contact time to retain chelating solutions in root canals for the removal of inorganic debris. One previous study found that 1-minute EDTA irrigation produced significantly more smear layer removal than 30-second or 15-second irrigation [16]. Although it has been suggested that EDTA has prejudicial effects on root dentine if applied for longer than 1 min [17], other studies have used root canal irrigants for 5 min without deleterious effects on root dentine [18,19].
Unlike these studies, we used irrigating solutions for a contact time of 2 min, which improved the solutions’ chelating capacity. The 0.3% chitosan solution was able to remove the smear layer with similar results to other solutions with higher concentrations. The chelating effect of chitosan observed in previous studies indicates that this solution acts on the inorganic portion of the smear layer [10]. This chelating behavior has been widely researched in industry for the recuperation of metal ions during waste-water treatment, and to reduce unwanted metals in drinking water [20]. Despite these demonstrable characteristics, the lowest calcium concentration in our study was obtained with 0.3% chitosan. This contrasts with the results of a previous study in which 15% EDTA and 0.2% chitosan groups contained the highest concentrations of calcium ions extracted from root canals, without significant differences between them [7]. A possible explanation could be factors influencing the agents’ demineralization capacity, such as contact time, pH, concentration, and the amount of available solution [17,21]. This might also be explained by the higher concentration of chitosan (0.3%) used in the present study. The present study used a 2.25% concentration of peracetic acid as in previous studies [1]. This solution is relatively cytotoxic, has antibacterial, sporicidal, antifungal, and antiviral effects and has been used for the elimination of biofilm formation in various areas [22,23]. It has been suggested that the acetic acid component is responsible for the dissolution of inorganic material. It also bonds to calcium to form complexes that are easily soluble in water [24]. However, it is relatively caustic when in contact with the oral mucosa. Another study adopted a protocol with a 3-minute peracetic acid treatment [1], while the present study applied the solution for 2 min in order to avoid introducing too many study variables. The standard chelating solutions assayed in this study – 20% citric acid and 17% EDTA – showed a similar capacity to remove the smear layer. In another SEM study, the cleaning capacity of 15% EDTA, 10% citric acid, 10% sodium citrate, apple cider vinegar, 5% acetic acid, 5% maleic acid and 1% sodium hypochlorite were measured, finding that 15% EDTA and 10% citric acid removed the smear layer from dentine walls completely [7]. An earlier study also used citric acid at the same concentration and for the same purpose and confirmed this capacity of citric acid [15]. Another study used a higher concentration – 20% – of citric acid observing that this concentration did not have any deleterious action on the surrounding tissues as it is not highly ionised [25]. For this reason, the present study used a 20% concentration of citric acid to boost its chelating capability. In relation to the cleaning effects on the three thirds assessed in SEM images, no significant differences were observed between the apical, middle and coronal thirds among any of the chelating solutions. In another study, 17% EDTA and Bio-pure MTAD were not able to remove the smear layer in the apical third [26]. This difference in results may be explained by the fact that in the present study the apices of the specimens were patent, so the chelating solution passed through the entire root canal during irrigation, passing directly into the container through the apical foramen. Therefore, the volume of solution that acted on all three parts was always the same.
Flame atomic absorption spectrophotometry (FAAS) indicated that all solutions were able to eliminate calcium ions from the root canal walls. The presence of calcium in the solution after irrigation does not only derive from decalcification of the smear layer’s inorganic structure; chelating and demineralizing solutions also act on the hydroxyapatite calcium matrix of the dentine, with subsequent collagen exposure and reduction of microhardness [27]. Another study evaluated the demineralizing effect of 15% EDTA, 15% citric acid, 5% phosphoric acid and 2.5% sodium hypochlorite on root dentine. The dentine specimens remained in the solutions for different lengths of time. The results showed that the highest calcium concentrations were extracted with 15% EDTA and 15% citric acid, without significant differences between the two agents [28]. These results converge slightly from the present study, which used citric acid at 20%, confirming the assertion made in an earlier study that the chelating action of citric acid becomes stronger as its concentration increases [29]. Within the limitations of the present work, 20% citric acid, 17% EDTA, 2.25% peracetic acid and 0.3% chitosan, with an application time of 2 min, effectively removed the smear layer from the middle and apical thirds of root canals. In addition, 20% citric acid, 17% EDTA and 2.25% peracetic acid produced the most effective demineralization of the root dentine, followed by 0.3% chitosan.
AcknowledgmentThe authors thank Dentsply-Maillefer for the donation of the instruments used in this study. The authors deny any conflicts of interest related to this study.
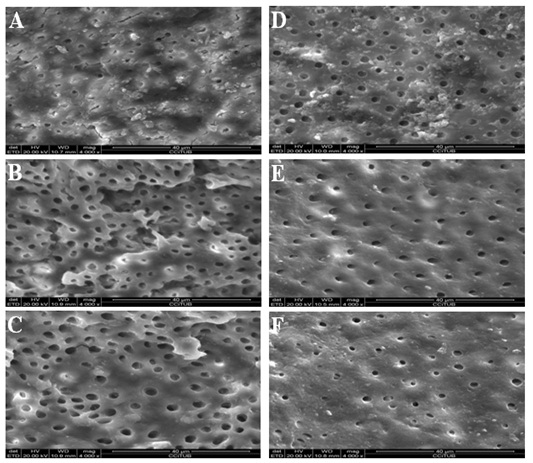
Figure 1: Scanning electron microscope photomicrographs (x4000 magnification). 0.3% Chitosan solutionirrigated group: (A) Coronal third. (B) Middle third. (C) Apical third. 20% citric acid solution-irrigated group: (D) Coronal third. (E) Middle third. (F) Apical third.
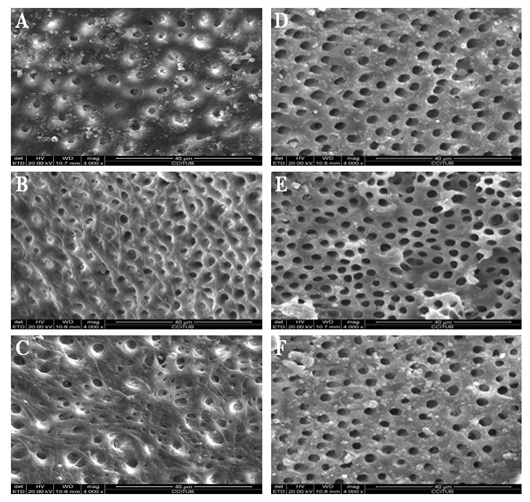
Figure 2: Scanning electron microscope photomicrographs (x4000 magnification). 2.25% peracetic acid solution-irrigated group: (A) Coronal third. (B) Middle third. (C) Apical third. 17% EDTA solution-irrigated group: (D) Coronal third. (E) Middle third. (F) Apical third.
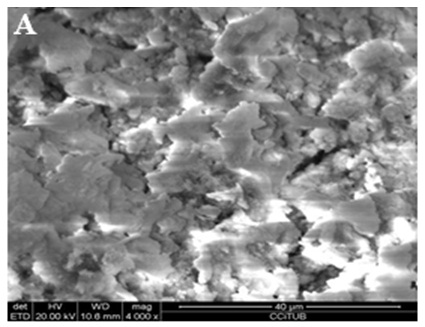
Figure 3: Scanning electron microscope photomicrographs (x4000 magnification). Ionized water solution-irrigated group: (A) Coronal third.
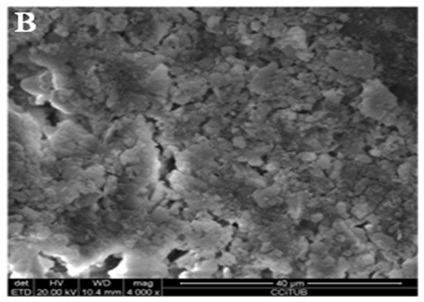
Figure 3B: Middle third.
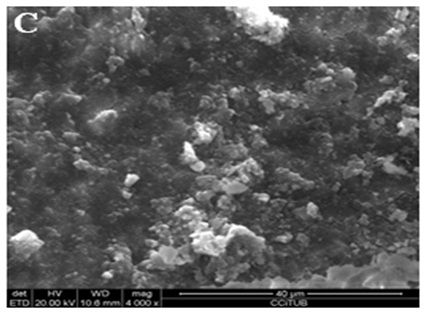
Figure 3C: Apical third.
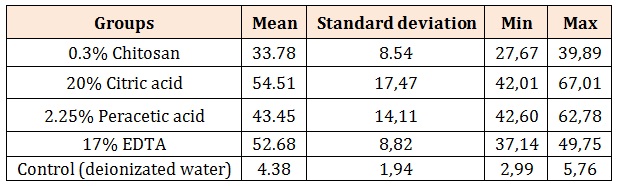
Table 1: Means and Standard deviations of the calcium ion concentration in the solutions assayed (ppm).
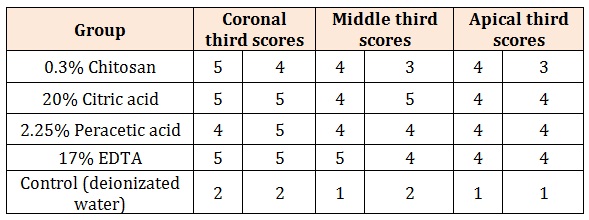
Table 2: Smear layer scores in the coronal, middle and apical thirds of the root canals in all study groups.
Chat with us on WhatsApp