Citation: Srividya L, et al. Hepatoprotective Activity of Hydroalcoholic Extract of Murraya Koeinigii Leaves against CCl4-Induced Hepatotoxicity in Rats. Adv Clin Toxicol 2017, 2(1): 000118.
*Corresponding author: Srividya Lonkala, Associate Professor, Jyothishmathi Institute of Pharmaceutical Sciences, Beside LMD Police Station, Nusthulapur, Karimnagar-505 481,India, Email: svps11288@gmail.com
The objective of the present study was to evaluate hepatoprotective activity of hydroalcoholic extract of Murraya koenigii leaves (HAMK) in rats. Hepatic damage was induced by administration of carbontetra chloride (1 ml/kg, b.w, p.o.) in combination with liquid paraffin (1:1) as a single dose on 7th day. The extent of liver damage was studied by estimating biochemical parameters. Administration of HAMK (100 mg & 200 mg/kg) for 6 days before carbontetrachloride administration showed a significant reduction (p < 0.01) of serum liver damage enzymes markers aspartate transaminase, alanine transaminase, total bilirubin and alkaline phosphatase (ALP). Histopathological changes of hepatic tissue were also evaluated in control and HAMK treated groups. Results also indicated that HAMK possessed potential antioxidant effect by increasing the levels of glutathione and also possessed free radical scavenging activities. The hepatoprotective activity of Murraya koenigii was comparable to standard drug silymarin (50 mg/kg).
Keywords: Murraya Koenigii; Hepatoprotective Activity; SGOT; SGPT
Liver is the major primary site for Biotransformation of drugs. Liver being the major detoxifying organ of the body, it is continuously exposed to xenobiotics and drugs [1]. Generally the prescribed drugs are tolerated but highdoses may commonly cause drug induced liver disease. Hepatotoxicity is one of the very common ailment resulting into serious debility even mortality [2]. Many hepatotoxic chemicals cause damage to the hepatic cells mainly by lipid peroxidation and other oxidative damage [3]. Modern, conventional or synthetic drugs used in the treatment of hepatic disease are inadequate and as there is no reliable liver protective agent, many Ayurvedic preparations, the Indian system of medicine, are recommended for the treatment of liver disorders [4]. Therefore, it is required to search for alternative drug with more safety and efficacy and replace the present drugs [5-6]. Herbal medicines play a major role in the management of various kinds of hepatic disorders. Natural remedies from medicinal plants are considered to be effective and safe alternative treatments for hepatotoxicity.
Murraya koenigii, commonly known as Curry leaves belonging to the family rutaceae is one such highly valued plant used for seasoning in Indian cooking. This plant has wide array of properties that include anti diarrhoeal, antiemetic, anti diabetic, antimicrobial, antiinflammatory, febrifuge and anti-ulcer activities [7-8]. However, there are only few scientific studies on hepatoprotective activity in the review literature. Hence, the present study was planned to evaluate the hepatoprotective activity of hydroalcoholic extract of Murraya koenigii leaves against carbon tetrachloride induced hepatotoxic study in Wistar rats.
Materials and MethodsCollection of plant material: The leaves of Murraya koeinigii used in the study were collected from local regions and authenticated by taxonomists. These were made free from the adherent foreign material, air-dried, cut in to small pieces and coarsely powdered mechanically.
Preparation of extract: Hydroalcoholic extract was prepared by the Soxhletion method. The powder of Murraya koenigii was extracted with 70% ethanol and 30% distilled water (i.e. hydroalcoholic extract) using soxhlet apparatus (50-550C) for three days [9]. The extract was concentrated in a ventilated oven at 45oC for 24 h. 50 g of dried powder yielded an extract of about 8 g which was dark brown in color. It was dissolved in 0.5% Carboxy-methyl-cellulose (CMC) before administering it to the experimental animals.
Chemicals: Carbon tetrachloride was purchased from (SD fine chem. Limited, Mumbai, India), Silymarin (Sigma, St Louis, USA), Sodium CMC (Central drug house, New Delhi), Formaldehyde solution (E-Merck, Mumbai, India), Glutathione (Himedia, Mumbai, India).
Phytochemical Screening: The hydroalcoholic extract of Murraya Koeinigii (HAMK) was screened for the presence of various phytochemical constituents like Carbohydrates, alkaloids, Tannins, steroids, Glycosides, Saponins and proteins & amino acids [10,11].
Experimental Animals: Male Wister rats of weighing 150–180 g (4–8 weeks) were used for the study and were procured from Mahaveera Enterprises, Hyderabad. They were housed in polypropylene cages and were maintained at room temperature of 23 ± 2°C and relative humidity 50% with 12:12 h light : dark cycle and free access to food and water ad libitum throughout the period of acclimatization and experimental study. All the experimental protocols were duly reviewed and approved by the Institutional Animal Ethics Committee.
Acute toxicity study: Acute toxicity studies were performed using male Wistar rats. The animals were fasted overnight prior to the experiment and maintained under standard laboratory conditions. HAMK was administered orally using various doses upto 2000 mg/kg and observed for the mortality and behavioral changes [12].
Study design: The rats were divided five groups of six each (n=6) and were treated as follows to evaluate the hepatoprotective activity of HAMK.
Group-I: Served as normal control and received 1ml/kg of 0.5% vehicle p.o. for 7 days.
Group-II: Served as disease control and received the vehicle for seven days followed by CCl4 and liquid paraffin solution (1:1) on the 7th day [13].
Group-III: Rats treated with HAMK at a dose of 100mg/kg for 7 days followed by CCl4 and liquid paraffin solution (1:1) administration p.o on the 7th day.
Group-IV: Rats treated with HAMK at a dose of 200mg/kg for 7 days followed by CCl4 and liquid paraffin solution (1:1) administration p.o on the 7th day.
Group-V: Served as standard group and received 50 mg/kg of Silymarin p.o. for 7 days followed by CCl4 and liquid paraffin solution (1:1) administration p.o. on the 7th day [14].
Collection of blood and liver tissue samples: Treatment with HAMK was continued for 7 days and on 8th day blood samples were collected under mild anesthesia by retro orbital puncture method. Serum was separated by centrifugation of blood samples at 10000 rpm for 10 minutes. The animals were then dissected; the livers were carefully removed, washed with 0.9% saline solution and preserved in formalin solution (10% formaldehyde) for histopathological studies.
Biochemical studies: To evaluate the hepatoptotective activity of HAMK, blood samples were estimated for various liver damage markers like alanine aminotransferase (ALT) or serum glutamate pyruvate transaminase (SGPT), aspartate aminotransferase (AST) or serum glutamate oxaloacetate transaminase (SGOT) were estimated using the kits (Crest biosystems, Goa, India), Total serum bilirubin (TB), serum alkaline phosphatase (ALP) using (Excel Diagostics, Hyderabad, India). To assess the antioxidant status, in all the treated groups we have estimated glutathione levels (GSH) by using the method of and total antioxidant status (TAS) by using Blios, 1958 [15,16].
Histopathology: Fresh liver tissues previously trimmed to approximately 2 mm thickness were placed in plastic cassettes and immersed in neutral buffered formalin for 24 h. The fixed tissues were processed routinely and then embedded in paraffin blocks, sectioned with microtome (0.7 μ thickness) deparaffinized and rehydrated using standard techniques. The extent of carbon tetrachlorideinduced liver injury was evaluated by assessing morphological changes in liver sections stained with hematoxylin and eosin using standard techniques and photographed.
Statistical analysis: All the experimental values were expressed as mean ± SD (n = 6). One-way ANOVA and Dunnett’s test were used to compare means from the control group and each of the groups exposed to toxicant and HAMK the statistical significance was judged at the 0.05 probability level.
Results and DiscussionPreliminary phytochemical screening of the HAMK revealed the presence of alkaloids, steroids, phenolics and tannins. No adverse effects and mortality of the animals were observed during the period of the study, 24 h up to the dose of 2000 mg/kg b.w. p.o. of hydroalcoholic extract of MK.
CCl4 induced hepatic injury is a commonly used model for the hepatoprotective drug screening. Rats treated with single dose of CCl4 developed significant hepatic damage as assessed from the elevated serum levels of SGOT, SGPT, ALP, and Total Bilirubin (Table-1). The results of the hepatoprotective activity of hydroalcoholic extract of MK were showed in table 1.
In contrast, groups treated with 100 and 200mg/kg of HAMK showed a significant dose dependant inhibition (p<0.01) of the elevated SGOT, SGPT, ALP, and Total Bilirubin levels induced by the CCl4 induction. This may suggest that treatment with HAMK accorded a protection against CCl4 induced increase in serum liver damage marker levels in a dose dependant manner. The degree of hepatoprotection seemed to increase with the increasing doses of HAMK (Table 1). The toxic effects of CCl4 was controlled in the animals treated with the hydro-alcoholic extract of Murraya Koeinigii by way of restoration of the levels of the liver function biochemistry similar to that of standard drug, Silymarin.
Administration of CCl4 to the rats resulted in the significant (p<0.01) reduction of reduced glutathione levels and total antioxidant status in blood. Similar to the Silymarin, treatment with HAMK at doses of 100 and 200mg/kg elevated these reduced levels to the near normal after 24 hrs. This indicates the antioxidant activity of HAMK will be the protective mechanism behind the hepatoprotective action of HAMK against CCl4 induced liver injury.
HistopathologyHistopathological profile of the animals showed in Figure 1. Liver tissue of control groups (A) showing normal hepatocytes, sinusoidal spaces, and central vein. Carbon tetrachloride treated (B) liver tissues showing intense centrilobular necrosis, vacuolization and macrovesicular fatty changes, sinusoidal congestion broad infiltration of kupffer cells, ballooning lymphocytic degeneration and loss of cell boundaries. The animals treated with hydro-alcoholic extract of Murraya Koeinigii (100 and 200 mg/kg) exhibited significant dose dependent liver protection against the toxicant as evident by the presence of mild to moderate hepatic cords, absence of necrosis and lesser fatty infiltration (Figure 1C and 1D). The sections of liver taken from the animals treated with standard drug silymarin (E) showing the normal hepatic architecture, which was similar to that of control.
The hepatoprotective activity of hydroalcholic extract of Murraya Koeinigii was also supported by the histopathological examinations of rat livers treated with CCl4 and HAMK. The hepatoprotective activity of many plants has been attributed to their high phenolic contents [17]. The phenolic compounds of HAMK may have attributed towards the hepatoprotective activity of extract because other studies have demonstrated that various phenolic compounds contain significant hepatoprotective activity [17].
ConclusionIn conclusion, the present study results indicated that under the present experimental conditions, hydroalcholic extract of Murraya Koeinigii showed a dose dependent hepatoprotective activity against carbon tetrachlorideinduced liver damage in rats.
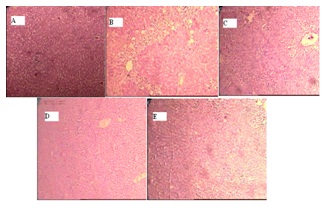
Figure 1: Histopathological evaluation of Hepatoprotective activity of hydro-alcoholic extract of Murraya Koeinigii in rats. (A) Normal (B) CCl4 treated (C) CCl4 treated +HAMK (100 mg/kg) (D) CCl4 treated +HAMK (200 mg/kg) (E) CCl4 treated +Silymarin.
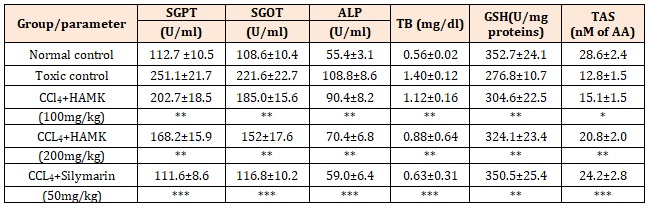
Table 1: Effect of hydroalcoholic extract of MK on various biochemical parameters in CCl4-induced Hepatotoxicity in rats.
All the values of Mean±SD; n=6; *p<0.05, **p<0.01, ***p<0.001 vs toxic control
Chat with us on WhatsApp