
Citation: Biswas D, et al. A Study of Pomegranate Fruit on Cisplatin- Induced Acute Renal Failure in Adult Bulb/C Mice. Adv Pharmacol Clin Trials 2016, 1(2): 000110.
*Corresponding author: Dipak Biswas, Pharmacology and animal cell culture Unit Laboratory Institute of Bioresources and Sustainable Development, India, Email:biswasdipak0@gmail.com
Aim: Study was undertaken to evaluate the effect of Punica granatum L. on cisplatin-induced renal failure in adult Bulb/c mice.
Materials and Methods: Gr.-I rats served as normal, received 0.5 ml of 5% Tween-80 in distilled water, Gr.-II was injected with Gentamicin (100 mg/kg, ip), Gr.-III was injected with Gentamicin and selenium (2 mg/kg, ip), and Gr.-IV-IX were given orally Punica granatum fruit chloroform extract (PGCE) and Punica granatum methanol extract (PGME) at the doses of 100, 200 and 400 mg/kg, respectively, were administered for three days in cisplatin-induced renal failure in adult Bulb/c mice. On last day, Blood and 24h urine was collected and used for estimation of serum and urine creatinine, urea, uric acid levels. The kidney homogenate was used for the estimation of LPO, SOD, CAT and GSH levels and Kidney sections were analyzed for histopathology.
Results: Cisplatin caused significantly (p<0.001) decreased in the levels of SOD, CAT and GSH, when compare to normal group of mice. More over treatment with Punica granatum L. significantly increased the levels of SOD, CAT, GSH when compare to control group of mice. The Nephroprotective potential of Punica granatum L. can be attributed to its antioxidant property.
Conclusion: It is concluded that administration of PGCE and PGME extracts not only did not ameliorate nephrotoxicity induced by Cisplatin in adult Bulb/C mice, but also aggravated renal damage.
Keywords: Cisplatin; Punica granatum; Renal failure; Lipid peroxidation; Antioxidant
Cisplatin (Cis-diamminedichloroplatinum, CP) has been successfully used as a chemotherapeutic agent is a currently one of the most important chemotherapeutic drugs used in treatment of tumors, lung cancer, antineoplastic action, is related to its accumulation in the proximal tubule cells, such as hepatotoxicity, ototoxicity, neurotoxicity, gastrotoxicity, myelosuppression, and allergic reactions [1,2]. However, the clinical usefulness of this drug is limited due to nephrotoxicity induction, a side effect that may produce in various animal modules. Cisplatin gets accumulated in the kidney tubule, causing nephrotoxicity [3]. A number of plants have been used in India and elsewhere which claimed efficient cure of urinary stones [4]. In cold desert Punica granatum was documented the treatment of urinary disorder [5]. Synthetic or herbal agents have been used to investigated as supplementations against Cisplatin induced nephrotoxicity [6,7]. However, Previous studies showed that some antioxidant agents have protective effect against Cisplatin induced nephrotoxicity [8-10]. Herbs are after administrated in combination with chemical drugs, which may enhance the potential of herb-drug antioxidant activity. Several flavonoids, including quercetin and some other polyphenols are found in Morus alba, Zingiber, and garlic, which have been used against kidney toxicity induced by nephrotoxins. Pomegranate has antioxidant, anti-inflammatory, and anti-microbial, urolithiasis effects [11-13,5]. Thus, the current study was designed to investigate whether methanol and chloroform extract of pomegranate fruit could have nephroprotective role against CDDP in adult Bulb/C mice.
Materials and MethodsPlant material
The Punica granatum fruit were collected from Bagalkot districts, region of North Karnataka. The Plant was authenticated at Department of Botany, B.V.V. Sangha’s Science College, and Bagalkot. A voucher specimen 23/2010 was deposited in the same institute. The fruits of Punica granatum was shade-dried and uniformly powdered and subjected to hot continuous solvent extraction with petroleum ether (40-60°C) to defat, followed by chloroform and methanol extraction. The solvent was completely removed by using rotary flash evaporator and dried in lyophilizer (Mini Lyotrap, Serial No. J8199/5, LET Scientific Ltd, UK) [14]. The percentage of extract yield of PG chloroform and methanol extracts was 3.23% and 5.31%, respectively. It was calculated in terms of dried weight. These dried powdered extract were formulated as suspension in distilled water using 5% Tween-80 as suspending agent. All the chemicals were purchased from Hi-media, Mumbai and Sigma Chemicals Co St Louis, USA and were of analytical reagent grade.
In-Vivo Cisplatin induced nephrotoxicity in adult Bulb/C mice:Experimental animals: Experiments were performed in accordance with the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA). The experimental protocol in the study was approved by the Institutional Animals Ethical Committee (HSKCP/IAEC, Clear/2010-11). The Bulb/c mice of male sex (25-35g) were obtained from the central animal house of H. S. K. College of Pharmacy and Research Centre, Bagalkot. The animals were housed at room temperature (22-28 ºC) for 12 hr dark and 12 hr light cycle and given standard laboratory feed and water ad-libitum. Cisplatin induced nephrotoxicity model (Nair et al) was used to assess the nephroprotectivity activity in Bulb/c mice [3].
Experimental design: Mice were divided into 9 groups each contain 6 animals, Group 1: served as normal, received 0.5 ml of 5% Tween-80 in distilled water treated orally for 3 days. Group 2: served as control, received 0.5 ml of 5% Tween-80 in distilled water treated orally for 3 days and simultaneously injected ip with Cisplatin 12 mg/kg in normal saline. Group 3: Treated orally for 3 days with Selenium 2 mg/kg in distilled water and simultaneously injected ip with Cisplatin 12 mg/kg in normal saline. Standard drug Selenium was administered by oral gavage 1h before and 24 hrs and 48 hrs and 72 hrs after the cisplatin injection. Group 4, 5 and 6 treated orally for 3 days with PG Chloroform extract (PGCE) at a dose of 100, 200 and 400 mg/kg suspended in 0.5 ml of 5% Tween-80 in distilled water and simultaneously injected ip with Cisplatin 12 mg/kg in normal saline. Test sample PGCE were administered by oral gavage 1h before and 24 hrs and 48 hrs and 72 hrs after the cisplatin injection, respectively. Group 7, 8 and 9 treated orally for 3 days with PG Methanol extract (PGME) at a dose of 100, 200 and 400 mg/kg suspended in 0.5 ml of 5% Tween-80 in distilled water and simultaneously injected ip Cisplatin 12 mg/kg in normal saline. Test sample PGME were administered by oral gavage 1h before and 24 hrs and 48 hrs and 72 hrs after the cisplatin injection, respectively.
Biochemical parametersAnalysis of urine samples: All the animals were kept in individual all glass metabolic cages and the urine sample of 24 h was collected on 72 h of cisplatin injection and acidified by the addition of 3 N HCl, than centrifuged at 1500 rpm for 10 min. using refrigerated research centrifuge (Remi centrifuge instrument, Mumbai) to remove debris and supernatant were stored at -20°C until analyzed using reagent kits (ERBA diagnostics, Mannheim, GmbH, Germany).
Analysis of blood samples: 1.5 ml blood samples were collected by puncturing the retro orbital venous plexus from each animal in centrifuge tube without anticoagulant and allowed to clot at room temperature. The serum was separated by centrifugation at 1500 rpm for 15 min. in Remi’s refrigerated research centrifuge and used for estimation of serum creatinine, urea and uric acid using commercially available kits (ERBA, Diagnostic, Mannheim), using Star-21 plus semi-auto analyzer.
Analysis of kidney Sample: After 72 hrs last injection of cisplatin, mice were sacrificed under anesthesia and after dissection; both kidneys were removed and washed with cold 0.15 M KCl. The right kidney was minced with scissors homogenized in cold phosphate buffer (0.1 M, pH 7.4). The homogenate was centrifuged at 1500 rpm for 10 min at 4°C using Remi’s refrigerated research centrifuge, the kidney homogenate was used to estimate the biochemical parameters viz. Lipid peroxidation (LPO) was measured by the method of [15]. The antioxidant enzymes viz. Superoxide dismutase (SOD), Catalase and reduced Glutathione were estimated by the methods [16- 19].
Hispathological analysis: The left kidney was fixed in a 10% solution of buffered formalin (pH.7.4). The tissue was embedded in paraffin and the sections of 5 µm were taken using MAC microtome (Macro scientific works, Delhi) and stained with hematoxylin-eosin. The slides were examined for histological variations under microscope, Morphometric measurements were completed on Olympus PM-10ADS automatic light microscope (Olympus optical Co., Tokyo, Japan) with a 40X and 10X objective, calibrated ocular micrometer.
Statistical AnalysisAll the statistical comparison between the groups are made by means of One Way Analysis of Variance (ANOVA) and followed by Dunnett’s Multiple Comparison test. The P <0.05 regarded as significant using, Graph Pad Prism 5.01 Software (Graph Pad software, San Diego, CA, USA). The data expressed are Mean ± standard error of mean (S.E.M.).
ResultsEffect of PGCE and PGME on urine creatinine, BUN and uric acid levels in Cisplatin induced Nephrotoxicity in mice:
Creatinine: The urine creatinine (mg/dl) levels of different groups of mice is given in Table 1 and presented in Figure 1 this shows that urine creatinine of normal group of mice were mean ± SEM 18.93 ± 1.729 maintain in the normal range.
The urine creatinine levels of control mice were mean± SEM 33.24 ± 1.564 significantly (p < 0.001) increase than normal mice. The urine creatinine in cisplatin treated mice with Punica granatum L of chloroform and methanol extracts of different doses 100, 200, 400 mg/kg reduced to mean ± SEM 18.08 ± 2.292, 12.76 ± 0.7485, 15.74 ± 0.5282, 7.557 ± 0.88715, 9.963 ± 1.372, 15.81 ± 0.9787 respectively, there were significant (p<0.001 ) reduced in the urine creatinine against control group of mice. The creatinine level in Selenium 2 mg/kg treated in Cisplatin induced mice mean ± SEM 13.79 ± 0.7634 which significantly (p < 0.001) reduced in urine creatinine when compared to control group.
BUN: The urine BUN (mg/dl )levels of different groups of mice is given in Table 1 and presented in Figure 1 which shows that urine BUN of normal group of rats were mean ± SEM 36.93 ± 6.603 maintain in the normal range.
All values are mean ± SEM, (n = 6 animals) *p < 0.05, **p < 0.01, ***p < 0.001, Superscripts represents a= ***p < 0.001, b = **p < 0.01 as compare to control group by One Way Analysis of Variance Test (ANOVA) followed by Dunnett's Multiple Comparison Test. Punica granatum L. Chloroform Extract (PGCE). Punica granatum L. Methanol extract (PGME), ns = Non Significant.
The urine BUN levels of control rats were mean ± SEM 114.8 ± 1.287, significantly (p < 0.05) increase than the normal mice. The of urine BUN in Cisplatin treated rats with Punica granatum L. of chloroform 100, 200, 400 mg/kg and methanol extracts 100, 400 mg/kg reduced to mean ± SEM 99.42 ± 9.65, 34.87 ± 3.043, 143.8 ± 10.80 and 86.01 ± 14.90, 116.2 ± 13.55 respectively, there were significant (p < 0.01 ) reduced in the urine BUN against control group of mice. However, PGME 200 mg/kg shows non significant (p > 0.05) when compare to control group. The BUN level in Selenium 2 mg/kg treated in Cisplatin induced rats mean ± SEM 46.90 ± 3.996 which significantly (p < 0.001) reduced in urine BUN when compared to control group. The values are comparable to normal group.
Uric acid: The urine uric acid (mg/dl) levels of different groups of mice is given in Table 1 and presented in figure 1 which shows that urine uric acid of normal group of mice were mean ± SEM 3.790 ± 0.7938 maintain in the normal range. The urine uric acid levels of control mice were mean ± SEM 13.97 ± 1.456 which shows increase than the normal mice. The urine uric acid in Cisplatin induced mice with Punica granatum L. of chloroform and methanol extracts 100, 200, 400, mg/kg reduced to mean ± SEM 3.455 ± 0.6257, 9.701 ± 1.503, 4.323 ± 0.5509 and 8.498 ± 1.581, 4.561 ± 1.507, 5.592 ± 0.9344 respectively, there were significant (p < 0.001 ) reduced in the urine uric acid against control group of mice. The urine uric acid level in Selenium 2 mg/kg treated in Cisplatin induced mice mean ± SEM 4.399 ± 0.6363 which shows non significant (p > 0.05) reduced in urine uric acid when compared to control group. The values are comparable to normal group.
0.1276 and 1.574 ± 0.552 respectively, there were significant (p < 0.001 ) reduced in the serum creatinine against control group of mice. The creatinine level in Selenium 2 mg/kg treated in Cisplatin induced mice mean ± SEM 0.535 ± 0.097 which shows non significant (p < 0.05) reduced in serum creatinine when compared.
Effect of PGCE and PGME on serum creatinine, BUN and uric acid levels in Cisplatin induced Nephrotoxicity in miceCreatinine: The serum creatinine (mg/dl) levels of different groups of mice is given in Table 2 and presented in Figure 2 this shows that urine creatinine of normal group of mice were mean ± SEM 0.823 ± 0.123 maintain in the normal range.
All values are mean ± SEM, (n = 6 animals) *p < 0.05, **p < 0.01, ***p < 0.001, Superscripts represents a = ***p < 0.001 as compare to control group by One Way Analysis of Variance Test (ANOVA) followed by Dunnett's Multiple Comparison Test. Punica granatum L. Chloroform Extract(PGCE). Punica granatum L. Methanol extract (PGME), ns = Non significant.
The serum creatinine levels of control mice were mean ± SEM 2.645 ± 0.058 showing non significant (p < 0.05) increase than the normal mice. The serum creatinine in Cisplatin treated mices with Punica granatum L. of chloroform extracts 100, 200 mg/kg and methanol extract 400 mg/kg reduced to mean ± SEM 0.837 ± 0.139, 0.500 ± to control group.
BUN: The serum BUN (mg/dl) levels of different groups of mice is given in Table 2 and presented in Figure 2 this shows that serum BUN of normal group of mice were mean ± SEM 30.84 ± 3.981 maintain in the normal range. The serum BUN levels of control mice were mean ± SEM 92.14 ± 15.5 which shows increase than the normal mice. The of serum BUN in Cisplatin treated rats with Punica granatum L. of methanol extract dose 200 mg/kg reduced to mean ± SEM 173.0 ± 25.19 that was significant (p < 0.01 ) reduced in the serum BUN against control group of mice. However, PGCE 100, 200, 400 and PGME 100,400 mg/kg shows non significant (p > 0.05) when compare to control group. The BUN level in Selenium 2 mg/kg treated in Cisplatin induced mice mean ±SEM 92.14± 15.5 which shows non significant (p > 0.05) reduced in serum BUN when compared to control group.
Uric acid: The serum uric acid (mg/dl) levels of different groups of mice is given in Table 2 and presented in figure 2 which shows that serum uric acid of normal group of mice were mean ± SEM 3.790 ± 0.7938 maintain in the normal range. The serum uric acid levels of control mice were mean ± SEM 11.02 ± 1.364 which shows significantly (p < 0.001) increase than the normal rats. The serum uric acid in Cisplatin induced mice with Punica granatum L. of chloroform and methanol extracts of different doses 100, 200, 400, mg/kg reduced to mean ± SEM 7.658 ± 1.468, 4.111 ± 1.709, 1.930 ±0.8703 and 3.629 ± 1.816, 2.049 ± 0.3937, 1.354 ± 0.3293 respectively, there were significantly (p < 0.001) reduced in the serum uric acid against control group of mice. The serum uric acid level in Selenium 2 mg/kg treated in Cisplatin induced mice mean ± SEM 2.671 ± 0.5913 which shows significantly (p < 0.001) reduced in serum uric acid when compared to control group. The values are comparable to normal group.
Effect of PGCE and PGME on renal SOD, CAT, GSH, and LPO levels in Cisplatin induced Nephrotoxicity in miceLPO: The LPO TBARS (U/mg of protein) in the kidney of different group of mice are given in the Table 3 and presented in Figure 3.
The normal animal values were found to have mean ± SEM 179.1± 16.66 and control group shows mean ± SEM 375.7 ± 37.25 which is significantly (p < 0.01) higher when compared to normal group of mice. The Punica granatum L. of chloroform extract 400 mg/kg showing value reduce to mean ± SEM 136.8 ± 20.09 which significantly (p < 0.001) less when compared to control group of mice. However, PGCE 100, 200 and PGME 100, 200, 400 shown less significant (p < 0.01). The LPO level of standard drug Selenium 2 mg/ kg in Cisplatin induced nephrotoxicity treated mice mean ± SEM 147.6 ± 19.10 none significantly (p > 0.05) reduce in LPO when compared to control group of mice. From the above value it was formed that the group treated with Punica granatum L. and standard drug reduce the LPO level and the value are comparable to normal values.
SOD: The SOD (U/mg of protein) in the kidney of different group of mice are given in the Table No.3 and presented in Figure 3 The normal animal values were formed to have mean ± SEM 1454 ± 129.8 and control group shows mean± SEM 814.1 ± 39.06 which is significantly (p < 0.001) lower when compared to normal group of mice. The Punica granatum L. chloroform and methanol extract of 400 mg/kg showing value reduce to mean ± SEM 1420 ± 153.1 and 1470 ± 111.8 respectively, which significantly (p < 0.01) increased when compared to control group of mice. The SOD level of standard drug Selenium 2 mg/ kg in Cisplatin induced nephrotoxicity mice mean ± SEM 1676 ± 245.4 showing non significant (p > 0.05) when compared to control group of mice. From the above value it was formed that the group treated with Punica granatum L and standard drug increase the SOD level and the value are comparable to normal values.
CAT: The CAT (U/mg of protein) in the kidney of different group of mice are given in the Table No.3 and presented in Figure 3 The normal animal values were formed to have mean ± SEM 7.198 ± 0.1667 and control group shows mean± SEM 5.180 ± 0.3006 which is shows significantly (p < 0.001) less when compared to normal group of mice. The Punica granatum L. chloroform and methanol extract of 100, 200, 400 mg/kg showing value to mean ± SEM 8.339 ± 0.8808, 7.805 ± 0.6710, 6.736 ± 0.4533 and 1.847 ± 0.3826, 2.225 ± 1.064,1.059 ± 0.235 respectively, which significantly (p < 0.01) increased when compared to control group of mice. From the above value it was formed that the group treated with Punica granatum L and standard drug increase the CAT level and the value are comparable to normal values.
GSH: The GSH (U/mg of protein) in the kidney of different group of mice are given in the Table No.3 and presented in Figure 3 The normal animal values were found to mean ± SEM 22.10 ± 1.733 and control group shows mean ± SEM 6.259 ± 0.6198 which is shows significantly (p > 0.01) when compared to normal group of mice. The Punica granatum L. chloroform and methanol extract of different doses100, 200, 400 mg/kg shows value reduce to mean ± SEM 18.45 ± 2.669, 20.26 ± 2.029, 19.73 ± 2.992 and 16.69 ± 1.796, 20.16 ± 1.732, 20.41 ± 2.148 respectively, which significantly (p < 0.001) increased when compared to control group of mice. The CAT level of standard drug Selenium 2 mg/ kg in Cisplatin induced nephrotoxicity mice mean ± SEM 20.80 ± 3.339 showing significantly (p < 0.001) increase when compared to control group of mice. From the above value it was formed that the group treated with Punica granatum L. and standard drug increase the GSH level and the value are comparable to normal values (figure 4).
DiscussionThe traditional use of Punica granatum has been reported to regulate urine discharge and controls the burning sensation of urine [5]. In our study, we evaluated the following renal biomarkers for evaluation of renal damage: I. Renal hemodynamics by estimating serum and urine creatinine, urea and uric acid levels, II. Determination of antioxidant enzymes activities and lipid peroxidation in kidney homogenate, and III. kidney histopathology.
Nephrotoxicity is one of the major side effects of Cisplatin. Although several studies have been performed to elucidate the molecular mechanisms that cause Cisplatin nephrotoxicity, the factors responsible for this are not fully understood. Recently, induction of oxidative free radicals has been implicated in this process [20]. Different strategies have been proposed to inhibit Cisplatin induced toxicity. The development of therapies designed to prevent the damaging actions of free radicals may influences the progression of oxidative renal damage induced by Cisplatin.
The major antioxidant enzymes such as GSH, SOD and CAT were found to be decreased in Cisplatin treated animals and oral administration of Punica granatum L. of chloroform and methanol extract elevated these levels (Table 3). ROS such as hydrogen peroxide, the superoxide anion and hydroxyl radicals are generated under normal cellular conditions and are immediately detoxified by endogenous antioxidants, like GSH, CAT and SOD, but excessive ROS accumulation by Cisplatin causes an antioxidant status imbalance end leads to lipid peroxidation and GSH deplitation [21]. The basic effect of Cisplatin induced toxicity on the cellular membrane is belived to be peroxidation of membrane lipids. The depletion of glutathione at early intervals in treated animals may be due to its utilization in large amounts to combat the acute Cisplatin induced free radical damage, as glutathione is a major nonenzymatic antioxidant. The measurement of lipid peroxidation as thiobarbituric acid reacting substances (TBARS) is a convenient method to monitor oxidative damage in tissues. Reactive oxygen species cause peroxidation of membrane lipids with devastating effect on functional states. The preservation of cellular membrane integrity depends on protection or repair mechanisms capable of neutralizing oxidative reactions. Our data shows that Cisplatin induced LPO levels were significantly (p < 0.001) decreased by the oral administration of PGCE 400 mg/kg doses in chloroform extract. It also attenuated Cisplatin induced GSH deplitation in mice. It has been suggested that cisplatin is able to generate ROS and that it inhibits the activates of antioxidant enzymes in renal tissues [22]. In the present study the reduced activities of GSH, SOD and catalase in kidneys of mice treated with Cisplatin were restored by administration of PGCE and PGME to a considerable extent indicating the ability of PGCE and PGME to eliminate oxidative stress. Cisplatin preferentially accumulates in cells of the S3 segment of the renal proximal tubules and is toxified to form a reactive metabolite intracellularly by hydration. The primary symptoms of Cisplatin nephrotoxicity are inhibition of protein synthesis and intracellular GSH and protein-SH depletion, resulting in lipid peroxidation and mitochondrial damage [21]. The peroxidation of membrane lipids may account for its nephrotoxicity [23] Available evidence suggests that Cisplatin exert its nephrotoxic effects by the generation of free radicals. GSH and protein-SH from the major cellular anti-oxidant defense systems, which control lipid peroxidation. From these pathomechanisms of Cisplatin nephrotoxicity, it is clear that the nephrotoxicity of Cisplatin involves reactive radicals. Thus the reasonable cellular-protective agents against Cisplatin toxicity may have at least some anti oxidant properties to prevent GSH deplitation and scavenge the intracellular reactive oxygen species [24,25]. The present observations support the hypothesis that the mechanism of nephrotoxicity is related to the depletion of the antioxidant defense system. Cisplatin treatment has been shown to induce loss of copper and zinc in the kidneys. The decrease in sod activity in renal tissues following Cisplatin administration might be due to the loss of copper and zinc [26]. The activity of catalase and GSH is also found to decrease after Cisplatin administration resulting in the decreased ability of the kidney to scavenge toxic hydrogen peroxide and lipid peroxides. The results from the present study indicate that the PGCE and PGME extract significantly (p < 0.001) protect the depletion of GSH levels and antioxidant enzyme activity in the renal cortex of the mice treated with Cisplatin.
Hence, antioxidants and free radicals scavengers of natural and synthetic origin might provide nephroprotection in Cisplatin induced renal injury [27]. It has reported that the Punica granatum L contents Tannins, alkaloid, glycosides are major components [11]. The antioxidant properties of Tannins, glycosides at the present study mechanism of action is not known, it has been belived that Punica granatum L. contining potent antioxidant active constituent maybe.
ConclusionThe results of our study confirmed that chloroform extract of possesses potent free radical scavenging property. The present study demonstrates the potent antioxidant properties of the Punica granatum L. chloroform extract. Hence, it may be concluded that the mechanism of nephroprotection by Punica granatum L. of chloroform extract in Cisplatin induced mice be due to antioxidant activity.
AcknowledgementWe are thankful to Principal and Head, Department of Pharmacology, H.S.K. College of Pharmacy, Bagalkot, Karnataka, India, for providing necessary facilities and support and also extremely thankful to M/S Himalaya Drug Company for providing free drugs sample of Cystone for this study.
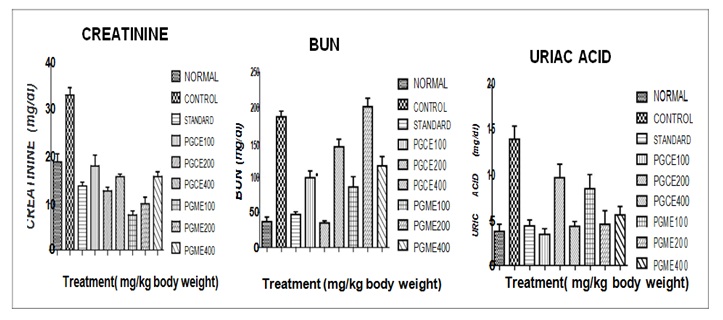
Figure 1: Effect of PGCE and PGME on urine creatinine, BUN, and uric acid levels in Cisplatin induced Nephrotoxicity in mice.
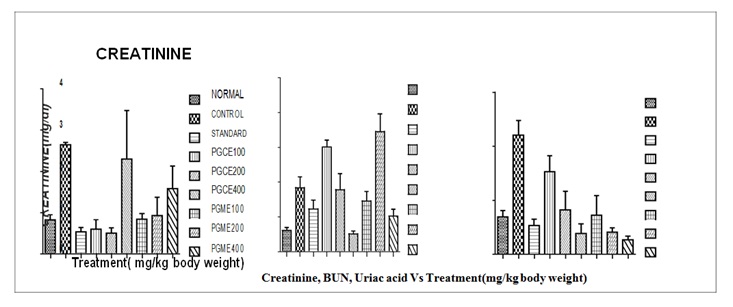
Figure 2: Effect of PGCE and PGME on serum creatinine, BUN and uric acid levels in Cisplatin induced Nephrotoxicity in mice.
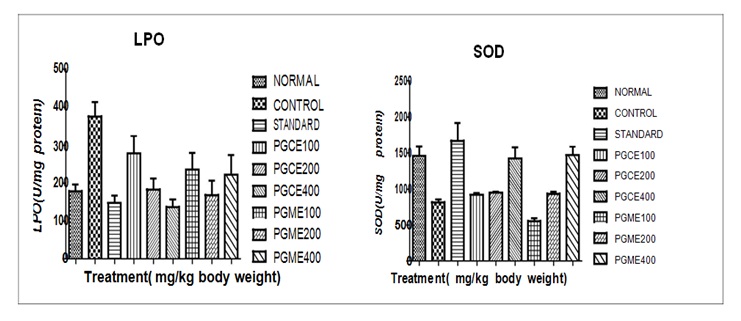
Figure 3: Effect of PGCE and PGME on renal LPO and SOD levels in Cisplatin induced Nephrotoxicity in mice.
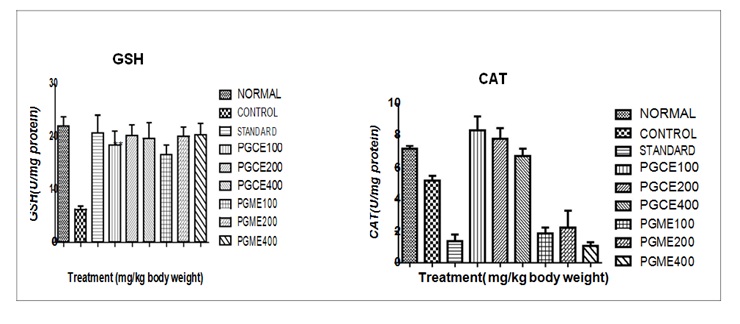
Figure 4: Effect of PGCE and PGME on renal LPO and SOD levels in Cisplatin induced Nephrotoxicity in mice.
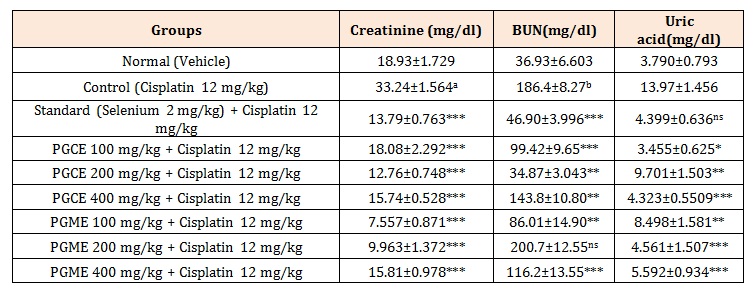
Table 1: Effect of PGCE and PGME on urine creatinine, BUN and uric acid levels in Cisplatin induced Nephrotoxicity in mice.

Table 2: Effect of PGCE and PGME on serum creatinine, BUN and uric acid levels in Cisplatin induced Nephrotoxicity in mice.

Table 3: Effect of PGCE and PGME on renal SOD, CAT, GSH, and LPO levels in Cisplatin induced Nephrotoxicity in mice.
Chat with us on WhatsApp