
Citation: Mervat El-Eshmawy, et al. Effect of Sitagliptin Monotherapy on Beta-Cell and Endothelial Functions in Patients with Newly Diagnosed Type 2 Diabetes. Diabetes Obes Int J 2017, 2(1): 000142.
*Corresponding author: Mervat M El-Eshmawy, Internal Medicine department, Specialized Medical Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt; Email: mervat2040@yahoo.com
Background/Aim: Recent research has focused on identifying new therapeutic targets and novel pathways for correcting impaired glucose homeostasis in type 2 diabetes (T2DM). The dipeptidyl peptidase-4 (DPP-4) inhibitor, sitagliptin contributes to suppression of postprandial hyperglycemia and diminishes risk of hypoglycemia through increasing the level of glucagon-like polypeptide. This study was conducted to assess the effect of sitagliptin on β-cell and endothelial functions in Egyptian patients with newly diagnosed T2DM.
Methods: The study enrolled 40 patients with newly diagnosed T2DM and 40 controls matched for age and sex. Anthropometric measurements, plasma glucose, lipid profile, LFTs, HOMA-IR, HOMA-β, amylase, lipase and flow mediated dilatation (FMD) were assessed. Patients with T2DM were evaluated before and after treatment with 100 mg daily sitagliptin for 24 weeks.
Results: Sitagliptin significantly reduced blood pressure, FPG, 2 h PPG, HbA1c, ALT, TGs, TC, LDL and HOMA-IR, whereas insulin, HOMA-β and FMD were significantly increased. Δ FMD was negatively correlated with Δ SBP, Δ FBG, Δ 2 h PPG, Δ HbA1c, Δ insulin, Δ TC, Δ TGs, Δ LDL, Δ HOMA-IR and positively correlated with Δ HOMA-β. With multivariate linear regression analysis, ΔTGs (B = – 0.140, P = 0.03), Δ LDL (B = – 0.158, P = 0.02), Δ HOMA-IR (B = – 0.904, P = 0.02) were independent predictors of Δ FMD after adjustment of Δ SBP, ΔTC.
Conclusion: Sitagliptin monotherapy is effective not only on glycaemic control and insulin sensitivity but, also it ameliorates endothelial dysfunction, blood pressure and dyslipidemia in newly diagnosed T2DM.
Keywords: Sitagliptin monotherapy; Endothelial function; Newly diagnosed T2DM.
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease attributed to genetic and environmental susceptibility. Insulin resistance and β-cell dysfunction are both responsible for pathogenesis of T2DM [1]. Endothelial dysfunction is a key event in the pathogenesis of diabetic micro and macrovasculopathy and has gained increasing attention in the study of diabetes associated cardiovascular complications [2]. The hallmark of endothelial dysfunction is the impaired nitric oxide (NO) bioavailability due to reduced production by NO synthase (eNOS), increased breakdown by reactive oxygen species (ROS), or both [3]. The contributing factors underlying impaired endothelial function in diabetes are varied and commonly include metabolic abnormalities such as hyperglycemia, excess liberation of free fatty acids (FFAs) and insulin resistance [4].
Several pharmacological agents that target either the relative insulin deficiency or insulin resistance in patients with T2DM are available. However, these agents have several limitations, such as less optimal control of postprandial hyperglycemia, increased risk of hypoglycemia, weight gain, gastrointestinal side effects and oedema [5]. Recent research has focused on identifying new therapeutic targets and novel pathways for correcting impaired glucose homeostasis [6]. The development of incretin mimetic and incretin enhancer drugs represent a promising therapeutic tool to achieve treatment goals, considering that people with T2DM have a significant decrease of incretin effect. Glucagon like peptide-1 (GLP-1), a prominent active compound of the incretin family, modulates many processes in pancreatic islet. It potentiates insulin synthesis and secretion, inhibits glucagon secretion, increases islet cell proliferation, decreases β-cell apoptosis, and also slows down gastric emptying [7].
Recently, Dipeptidyl-peptidase 4 (DPP-4) inhibitors; prevent the inactivation of incretins and increasing the endogenous active incretin levels; have attracted more and more attention, several of which have entered preclinical and clinical trials, and have received approval for use as an anti-diabetic agent. Sitagliptin was the first DPP- 4 inhibitor approved by the FDA. It is a potent, highly selective DPP-4 inhibitor, had a neutral effect on body weight, and contributes to suppression of postprandial hyperglycemia and to diminishes risk of hypoglycemia [8]. It leads to increases in insulin and C-peptide, reduction in glucagon, improvement in oral glucose tolerance and improvement in β-cell function and/or neogenesis [9]. GLP receptors are also located in myocardial, vascular endothelial and smooth muscle cells; GLP-1 induces improvements in the cardiac function, as well providing cardioprotective effects through the elevation of cyclic adenosine monophosphate (cAMP) levels, thus leading to a significant regression of arteriosclerotic lesions [10]. So, sitagliptin is expected to improve the endothelial dysfunction.
The aim of this study was to assess the effect of sitagliptin on β-cell function, endothelial function and some metabolic parameters in Egyptian patients with newly diagnosed T2DM.
Subjects and MethodsThis prospective study was included 40 patients (20 men and 20 women) with newly diagnosed T2DM; not controlled on life style management including diet and exercise; recruited from Outpatient Diabetes Clinic at Mansoura Specialized Medical Hospital. Those patients were evaluated before and after treatment with 100 mg once daily sitagliptin for 24 weeks. Also, 40 healthy subjects matched for age and sex were evaluated as controls. The study approved by the local ethical committee of institutional review board in Mansoura Faculty of Medicine, Internal Medicine Department. All participants signed an informed consent.
All participants were subjected to thorough medical history and clinical examination. Anthropometric measurements were obtained using standardized techniques; height was measured to the nearest 0.5 cm, body weight was measured to the nearest 0.1 kg, body mass index (BMI) was calculated as weight/height2 (kg/m2) and waist circumference (WC) was measured at the highest point of the iliac crest. Blood pressure was measured twice in the sitting position after 10 min of rest using a random-zero sphygmomanometer and the average of the 2 measurements were used in the analysis.
The criteria for exclusion were type 1 diabetes, secondary diabetes, poorly controlled diabetes (HbA1c >10%, fasting blood glucose level < 300 mg/dl), microvascular and macrovascular diseases, hypertension or the use of antihypertensive medications, acute or chronic renal failure, persistently elevated liver enzymes>3 folds, connective tissue disorders, malignancies, pregnancy, women taking birth control pills or hormone replacement therapy and smoking. We also excluded subjects who were drugs known to influence endothelial function i.e. antioxidants, aspirin, lipid lowering drugs.
Laboratory assayThe laboratory data including albumin, total bilirubin, aspartate transaminase (AST) and alanine transaminase (ALT), fasting plasma glucose (FPG), 2h post load plasma glucose (2h PPG) and creatinine were performed on the Dimension Xpand plus chemistry analyzer using its commercial kits (supplied by Siemens Technology, Princeton, New Jersey, USA). Hemoglobin A1C (HbA1c) was measured as an index of metabolic control on a DCA 2000 analyzer, fast ion exchange resin ( Roche Diagnostic, Germany). The reference range was 4.4% to 6.4%. Plasma insulin was assayed by an enzyme-linked immunosorbent assay (ELISA) insulin quantitative BIOS kit (Chemux BioScience, Inc, USA). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated with the formula: HOMA-IR = (Fasting insulin in mU/L X fasting plasma glucose in mg/dl)/ (405) [11]. Homeostatic model assessment Beta cell % (HOMA-β), a marker of basal insulin secretion of pancreatic β-cells, was calculated with the formula: HOMA-β cell % = (360 × fasting insulin in mU/L)/(fasting glucose in mg/dl – 63) [11]. Total cholesterol (TC), triglycerides (TGs), and high-density lipoprotein cholesterol (HDL-C) were estimated by commercially available kits (supplied by Spin react (Spain). Low-density lipoprotein cholesterol (LDL-C) was calculated according to Friedewald et al. [12].
Flow mediated dilatation (FMD)FMD was performed using high resolution ultrasound probe (Toshiba Powervision 6000 with a 7.5 MHz linear array transducer). Subjects were examined in a quiet, temperature-controlled room while they were fasting for 8-12h and lying in a supine position for 10 minutes with their arms resting on a comfortable position in a cradle support to ensure stable conditions during scanning. The right brachial artery was scanned over a longitudinal section, 3-5 cm above the right elbow. The interface between the near and far arterial wall and the vessel lumen has to be clearly defined. Lumen diameter was measured as distance between media-adventitia interfaces of far and near wall (baseline diameter). The cuff was inflated to ≥ 50 mmHg above systolic pressure for 5 minutes to occlude arterial inflow. On cuff deflation, reactive hyperaemia in the brachial artery occurs and results in vasodilatation if endothelial function is intact. The second scan was taken 60-120 seconds after the rubber cuff had been deflated to assess hyperemia diameter or post reactive diameter. FMD was reported as the percentage change from the baseline diameter. Value of FMD was presented as: [(post reactive diameter– baseline diameter)/baseline diameter) x 100].
Statistical MethodsData entry and analysis were performed using SPSS statistical package version 20. The data were expressed as mean ± SD for normally distributed data, frequency and proportion for categorical data and median (minimummaximum) for skewed data. Statistical comparison between 2 groups was assessed by Student’s t-test for parametric data, Mann Whitney (M.W.) for nonparametric data independent group and Wilcoxon signed rank test for nonparametric data dependent group. A chi-square test was used to compare categorical data. Pearson and Spearman correlations were used to study the relation between continuous variables. Multiple regression analysis was performed with Δ FMD as the dependent variable. SBP, TC, TGs, LDL and HOMA-IR were entered in the regression model. Δ: post treatment – pretreatment, P ≤ 0.05 was considered as significant.
ResultsTable1 presents the baseline characteristics of the study subjects. Patients with newly diagnosed T2DM had significantly higher systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, WC, ALT, FPG, 2 h PPG, HbA1c, fasting insulin, HOMA-IR (p < 0.001) and TGs (P < 0.001) than did healthy controls, whereas they had a significantly lower HDL and HOMA-β (P < 0.001) than did healthy controls. Patients with newly diagnosed T2DM had significantly lower FMD than did healthy controls (13.32 ± 5.39 vs. 23.6 ± 4.69, P < 0.001) (Figure1).
Treatment with 100 mg daily sitagliptin for 24 weeks significantly reduced SBP, DBP, FPG (P < 0.001), 2 h PPG (P < 0.001), HbA1c (P < 0.001), ALT (P = 0.02), TGs (P < 0.022), TC (P < 0.028), LDL (P = 0.009) and HOMA-IR (P < 0.001), whereas fasting insulin (P < 0.001) and HOMA-β (P < 0.001) were significantly increased. However, there were not statistically significant differences in weight, BMI, WC, albumin, bilirubin, AST, creatinine, HDL, amylase and lipase before and after treatment with sitagliptin. The percentage of endothelial dysfunction (FMD < 10%) was significantly decreased after treatment with sitagliptin [37.5 % (15/40) vs. 5 % (2/40), P = 0.001] (Table 2). FMD was significantly increased after treatment with sitagliptin in patients with newly diagnosed T2DM (13.32 ± 5.39 vs. 21.6 ± 7.42, P < 0.001) (Figure1).
Δ FMD was negatively correlated with Δ SBP (r = – 0.342, P = 0.03), Δ FBG (r =-0.361, P = 0.02), Δ 2 h PPG (r =-0.352, P = 0.02), Δ HbA1c (r =-0.320, P = 0.04), Δ insulin (r =-0.332, P = 0.03), Δ TC (r =-0.330, P = 0.03), Δ TGs (r =- 0.345, P = 0.02) and Δ LDL (r =-0.373, P = 0.01) (Table 3). Δ FMD was positively correlated with Δ HOMA-β (r = 0.329, P = 0.03) and negatively correlated with Δ HOMAIR (r =-0.380, P = 0.01) (Figure 2 and 3).
DiscussionIn patients with newly diagnosed T2DM, 100 mg daily sitagliptin treatment for 24 weeks significantly decreased FBG, 2h PPG, HbA1c. These results are consistent with many reports which studied the effect of sitagliptin on glycaemic homeostasis [13-15]. Surprisingly, Yanai et al. [16] found no significant difference in plasma glucose after 6-month of sitagliptin treatment, this study can be criticized by non-compliance of participant individuals as HbA1c is decreased while no change in plasma glucose.
In the current study, fasting insulin levels and HOMA-β increased and HOMA-IR decreased after treatment with sitagliptin in patients with newly diagnosed T2DM. Increase fasting insulin in the context with lowered blood glucose level; suggest an augmentation of insulin response. Accordingly, Scott et al. [17] and Drucker et al. [18] showed an improvement of HOMA-β associated with enhanced insulin release in the fasting state after treatment with sitagliptin. Also, Mohan et al. [19] and Tremblay et al. [20] demonstrated a larger increase in HOMA-β and a decrease in HOMA-IR from baseline in patients treated with sitagliptin compared with placebogroup suggesting an improvement in insulin sensitivity with sitagliptin treatment. In contrast, Raz et al. [9] and Tremblay et al. [20] found no significant effect of sitagliptin treatment on insulin level. Raz et al. [9] observed improvements in HOMA-β, but there was no significant effect of sitagliptin treatment compared with placebo on HOMA-IR, suggesting that sitagliptin may not affect peripheral insulin sensitivity.
In patients with T2DM, the incretin response and the following insulin secretory response after oral glucose is typically decreased by 50% compared with healthy control subjects. These observations suggest that deficient incretin secretion may be a critical point in the pathogenesis of T2DM [21]. Sitagliptin is one of the DPP-4 inhibitors which prevent the inactivation of incretins; increasing the endogenous active incretin levels. Incretins stimulate insulin secretion from pancreatic β-cells in glucose dependent manner, which is favorable for the treatment of diabetes [16]. Incretins can improve insulin sensitivity both locally (β-cells) and systemically (liver and muscle). These beneficial effects of sitagliptin on β- cell function are mediated via a direct action on β-cells by enhancing proliferation, neogenesis, and decrease the apoptosis and/or an indirect one by reducing the circulating levels of glucose and FFAs and, in consequence, glucolipotoxicity [22]. Also, they facilitate the uptake of glucose by muscles and liver while simultaneously suppressing glucagon secretion by α cell of the islets, leading to reduced endogenous production of glucose from hepatic sources [18].
We observed a significant decrease in TC, TGs and LDL after treatment with sitagliptin, whereas no significant change was detected in HDL. Similarly, Horton et al. [23] showed an improvement in lipid parameters from baseline in the sitagliptin group, with the exception of HDL, which remained unchanged. However, Derosa et al. [24] noted that sitagliptin decreased TC, LDL, TGs and increased HDL. In contrast, Kubota et al. [14] confirmed no significant changes in the lipid profile after 50 mg/day sitagliptin treatment for 12 weeks; the difference in dose and duration of sitagliptin treatment may explain this discrepancy. Improvement of lipid profile may be due to the improvement in glycaemic control and insulin resistance. Also, delayed gastric emptying [25] can be another proposed mechanism by which the incretins could reduce lipemia.
Our study revealed significant reductions in SBP and DBP after treatment with sitagliptin in patients with T2DM. In agreement, Takeda et al. [26] and Sakamoto et al. [27] concluded that both SBP and DBP were significantly decreased after treatment with sitagliptin. In addition, Mistry et al. [28] confirmed that sitagliptin treatment was associated with a small but statistically significant (2-3 mmHg) decrease in SBP evaluated by 24- hour ambulatory monitoring. However, Kubota et al. [14] found non-significant reduction in BP with sitagliptin. This is could be explained by the short term duration of this study. GLP-1(7–36) exhibits vascular actions via GLP- 1 receptor signaling and GLP-1(9–36), a metabolite of GLP-1 (7–36), has vasodilator effects independent of the GLP-1 receptor. DPP-4 inhibition within the microcirculation relaxes vascular tone mediated by NO causing peripheral vasodilatation and decreased peripheral vascular resistance [29]. The impact of incretin-based therapy on BP appears to be also related to activation of GLP-1 receptors in renal tissue, which results in decreased expression of the sodium-hydrogen transporter type 3 and leads to increased dieresis and sodium excretion in proximal renal tubules [30].
We noted that patients with T2DM had significantly elevated ALT compared with healthy controls; ALT was significantly decreased after treatment with sitagliptin. The most common cause of a mild elevation of ALT is nonalcoholic fatty liver disease (NAFLD) [31], which is the most prevalent liver disease in T2DM. A central abnormality in the pathogenesis of steatosis appears to be insulin resistance resulting in lipolysis, which increases circulating free fatty acids, which are then taken up by the liver as an energy source [32]. In parallel, Nauck et al. [33] found a slight decrease in ALT from baseline after treatment with sitagliptin for 52 weeks in 1172 patients. This modest reduction in ALT with sitagliptin suggests that a decrease in hepatic fat may have occurred. However, we did not perform hepatic imaging to ascertain this possibility, so the need for additional studies may be warranted. In contrast, Arase et al. [34] revealed no significant changes of average AST and ALT levels on sitagliptin treatment in patients with T2DM and NAFLD. So they speculated that sitagliptin is effective and safe for the treatment of T2DM complicated with non-alcoholic fatty infiltration in the liver.
In the present study, we observed that FMD was lower in patients with newly diagnosed T2DM than in healthy controls. In accordance, Henry et al. [35] concluded that T2DM is independently associated with impaired FMD. Su et al. [36] also assessed endothelial dysfunction in early stages of DM via FMD in 30 impaired fasting glucose (IFG) patients, 38 impaired glucose tolerance (IGT) patients, and 44 T2DM patients, they found that subjects with IFG and IGT had impaired FMD compared with subjects those with normal glucose tolerance, FMD was also impaired in patients with T2DM than those with IFG and IGT. Interestingly, even in normoglycemic subjects who are liable to develop DM, impaired endothelial dysfunction has been observed during an OGTT. This has led to the hypothesis that endothelial dysfunction may precede the development of overt DM, and that a repeated and prolonged exposure to postprandial hyperglycemia may play a significant role in the development of atherosclerosis [37].
Our findings revealed a significant improvement in endothelial dysfunction after treatment with sitagliptin; FMD was significantly increased to the point that it is not significantly different than the FMD of healthy controls which is surprising. In multiple regression analysis, each of ΔTGs, Δ LDL and Δ HOMA-IR were negative independent predictors of Δ FMD.
In support, many studies reported improvement of endothelial dysfunction with sitagliptin treatment [14, 38- 40]. The independent associations of FMD with TGs have been previously reported [41, 42]. Transient triglyceridemia decreases vascular reactivity, presumably by both endothelium-dependent and endotheliumindependent mechanisms [43]. Post-prandial lipaemia, with the production of TGs-enrichment of VLDL, results in endothelial dysfunction by an oxidative stress mechanism in patients with T2DM. Hence, exaggerated post-prandial lipaemia associated with reduced HDL-C may be important in the pathogenesis of vascular disease, particularly in T2DM [44]. Elevated levels of LDL were also found to be a major risk factor for atherosclerosis [45]. Moreover, oxygen-derived free radicals mediate the disruption of the endothelial cell surface layer and increase vascular wall adhesiveness by oxidized LDL [46]. In previous studies, HOMA-IR has been reported to be an independent predictor of brachial artery FMD [47,48]. Insulin resistance is frequently associated with endothelial dysfunction [49]. Normally the balance between NO-dependent vasodilator action and ET-1- dependent vasoconstrictor action of insulin is regulated by phosphatidylinositol 3-kinase-dependent (PI3K) and mitogen-activated protein kinase (MAPK)-dependent signaling in vascular endothelium, respectively. During insulin-resistant conditions, pathway-specific impairment in PI3K-dependent signaling may cause imbalance between production of NO and secretion of ET-1 and lead to endothelial dysfunction [50].
In multiple regression model, R2 = 0.46% suggesting that about 54% of improvement in FMD can’t be explained by improvement in TGs, LDL and HOMA-IR. This improvement may be due to a direct effect of sitagliptin on FMD or indirect effect through unmeasured variables in our study such as; adiponectin [14,51] and plasma levels of asymmetric dimethylarginine (ADMA) [52].
We and others [53,54] found that sitagliptin mostly have a neutral effect on body weight. However, Katsuyama et al. [55] confirmed that body weight significantly decreased by 1.5 kg after sitagliptin treatment for 6 months in obese group, while body weight did not change in non-obese group.
In the current study, amylase and lipase enzymes were significantly unchanged after sitagliptin treatment; suggesting that this drug may not be a risk factor for development of pancreatitis. These results are in parallel with Monami et al. [56] who conducted a meta-analysis of 134 eligible trials of patients with T2DM using sitagliptin for 12 weeks or more. This meta-analysis does not suggest any increase in the risk of pancreatitis with sitagliptin use or other DPP4 inhibitors. However, Elashoff et al. [57] reported a six fold increased risk of pancreatitis in patients using sitagliptin compared to users of other antidiabetic therapies. This study was strongly criticized as it was based on selected data retrieved between 2004 and 2009 from the FDA’s Adverse Event Reporting System [58].
ConclusionSitagliptin monotherapy is an attractive therapeutic strategy with a good safety profile for patients with newly diagnosed T2DM; it is effective not only on glycaemic control and insulin sensitivity but, also it ameliorates endothelial dysfunction, blood pressure and dyslipidemia. Further long term studies may be warranted for better evaluation.
Competing InterestsThe authors declare that they have no competing interests. This research did not receive any specific grant from any funding agency in the public, commercial or notfor-profit sector
Authors' ContributionsASN and MME drafted the manuscript, MME carried out flow mediated dilatation tests, OSS carried out the laboratory studies, NMS conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
AcknowledgmentThe authors thank all sample donors for their contribution to this study and all members of the Endocrinology Unit, Specialized Medical Hospital, Mansoura, Egypt.
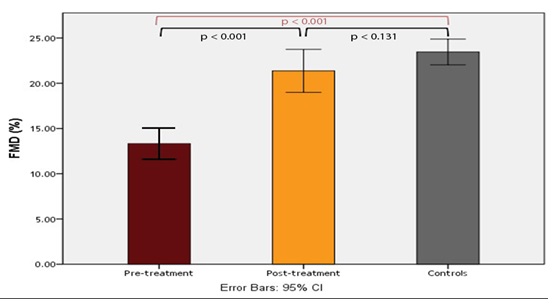
Figure 1: FMD in patients with T2DM (before and after sitagliptin treatment) and controls.
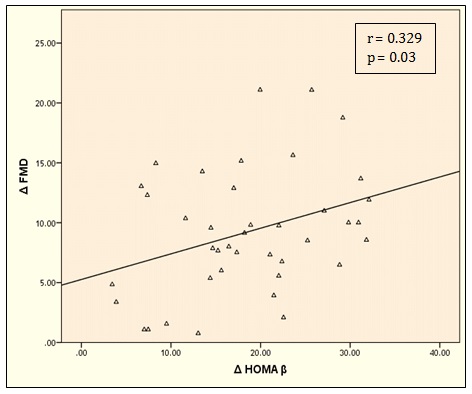
Figure 2: Correlation between Δ FMD and Δ HOMA-β.
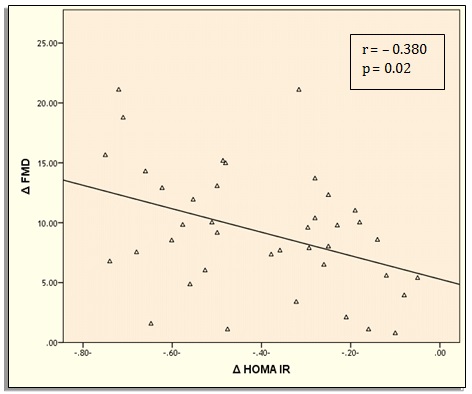
Figure 3: Correlation between Δ FMD and Δ HOMA-IR.
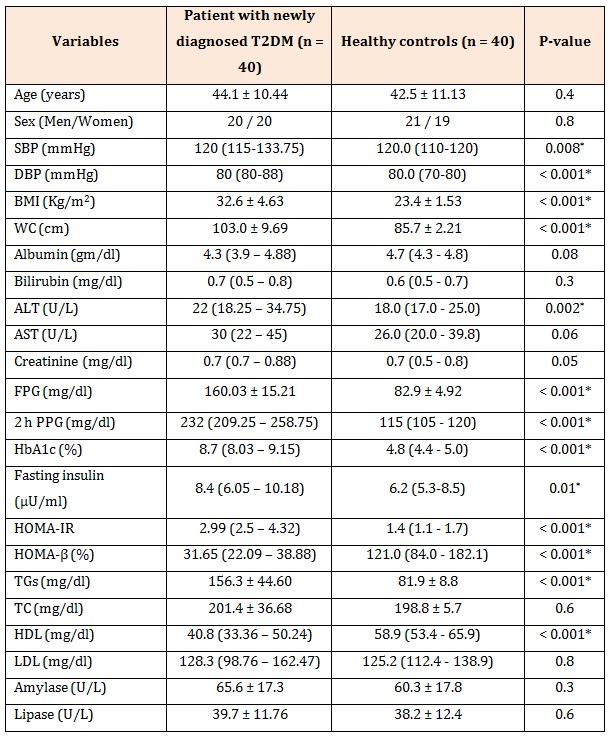
Table 1: Characteristics of patients with newly diagnosed T2DM (before treatment) and healthy controls.
Data are presented as mean ± SD and median (minimum-maximum) ,SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index, WC: waist circumference, ALT: alanine transferase, AST: aspartate transferase, FBG: fasting plasma glucose, PPG: post prandial plasma glucose, HbA1c: hemoglobinA1c, TGs: triglycerides, TC: total cholesterol, HDL: high density lipoprotein, LDL: low density lipoprotein, HOMA-IR: homeostasis model assessment of insulin resistance, HOMA-β: homeostasis model assessment of beta cell function,*P is significant if ≤0.05.
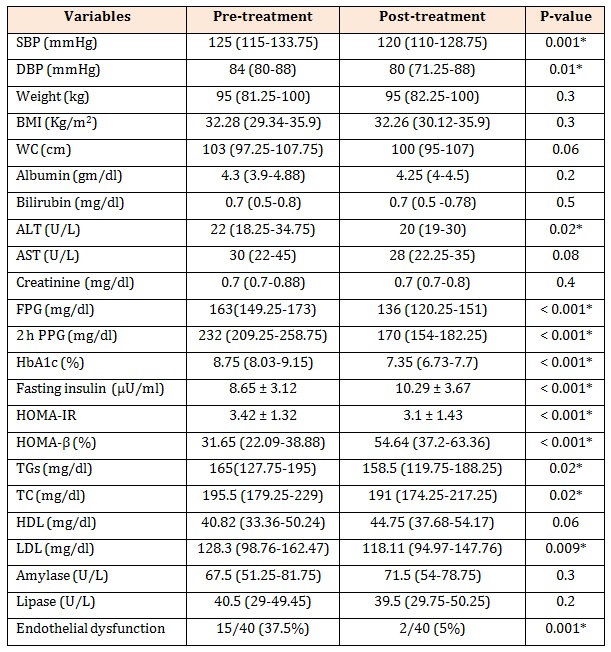
Table 2: Effect of sitagliptin treatment on studied variables in patients with T2DM.
Data are presented as mean ± SD and median (minimum-maximum), SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index, WC: waist circumference, ALT: alanine transferase, AST: aspartate transferase, FBG: fasting plasma glucose, PPG: post prandial plasma glucose, HbA1c: hemoglobinA1c, TGs: triglycerides, TC: total cholesterol, HDL: high density lipoprotein, LDL: low density lipoprotein, endothelial dysfunction: FMD < 10%,*P is significant if ≤0.05.
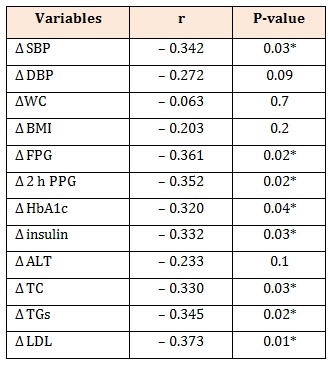
Table 3: Correlation between Δ FMD and Δ other variables in patients with newly diagnosed T2DM.
Δ: post treatment-pretreatment, FMD: flow mediated dilatation, SBP: systolic blood pressure, DBP: diastolic blood pressure, FBG: fasting plasma glucose, PPBG: post prandial plasma glucose, HbA1c: hemoglobinA1c, ALT: alanine transferase, TC: total cholesterol, TGs: triglycerides, LDL:low density lipoprotein, HOMA-IR: homeostasis model assessment of-insulin resistance, HOMA-β: homeostasis model assessment of beta cell function, P is significant if ≤0.05.
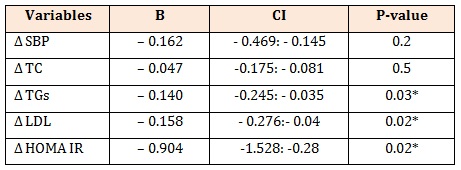
Table 4: Multivariate linear regression analysis with Δ FMD as dependent variable in patients with T2DM.
R2 = 0.461B: unstandardized coefficient, Δ: post treatment-pretreatment, SBP: systolic blood pressure, TGs: triglycerides, TC: total cholesterol, LDL: low density lipoprotein, HOMA-IR, homeostasis model assessment of-insulin resistance.
Chat with us on WhatsApp