
Citation: Saxena AK, et al. Current Concepts and Future Perspectives in the Optimization of Perioperative Blood Glucose Levels and Improvement of Outcomes. Diabetes Obes Int J 2017, 2(1): 000143.
*Corresponding author: Ashok Kumar Saxena, Professor and Incharge, Pain Clinic, Department of Anaesthesiology, University college of Medical Sciences and Guru Teg Bahadur Hospital, University of Delhi, India, Email: profashoksaxena@gmail.com
Diabetes is defined as a clinical syndrome consisting of constellation of signs and symptoms as a result of hyperglycemia, which results from absolute or relative deficiency of insulin, accompanied by absolute or relative excess of glucagon. Unfortunately, perioperative hyperglycemia has been associated with the greater risk of postoperative morbidity and mortality. On the other hand, hypoglycemia is equally dangerous. The adverse consequences of hypoglycemia include those on circulatory, autonomic, and central nervous systems Several studies have concluded that adequate glycemic control without excessive hyper- or hypoglycemia, has been associated with an improved surgical outcome. The controversy continues to exist in the context of establishment of target blood glucose levels and protocolization of the generalized indications for intensive insulin therapy initiation. This review article addresses the ever debatable issue on perioperative glycemic control in diabetic patients and focuses on recent advances and updates in the optimization of perioperative blood glucose levels, merits and demerits of various perioperative regimens, with recent studies on the effects of optimal perioperative glucose control on morbidly obese patients undergoing bariatric surgery, and additional recent studies on preoperative HbA1c and the associated risk of postoperative complications in patients undergoing oncosurgery. It has been recently observed that intra-operative hyperglycemia predisposed towards increase in the odds for postoperative hyperglycemia Therefore, an early and aggressive management of raised intra operative glucose levels, results in lower starting blood glucose concentrations for postoperative glucose management. The age and BMI also have significant positive effects on raised postoperative glucose levels. Also, initiation of insulin infusion early, at or more than 140 mg/dl blood glucose levels, does not increase the incidence of hypoglycemia. In conclusion, a protocol for perioperative regime should be flexible and common sense must be applied at every step during the perioperative management of a diabetic patient scheduled to undergo a major surgery
Keywords: Hyperglycemia; Cardiacdysautonomia; Glycosylated Haemoglobin; Random plasma glucose; Glucocorticoids
Diabetes is defined as a clinical syndrome consisting of constellation of signs and symptoms as a result of hyperglycemia, which results from absolute or relative deficiency of insulin, accompanied by absolute or relative excess of glucagon. As per the American diabetes Association 2013 guidelines, Diabetes can be diagnosed on the basis of Fasting plasma Glucose ≥126 mg/dl or Plasma Glucose after 2 hour oral glucose tolerance test (OGTT)≥200mg/dl or Glycosylated Hemoglobin (HbA1c)≥6.5% or Random plasma glucose ≥200 mg/dl plus symptoms of diabetes ( polyuria, polyphagia and polydipsia).
Hyperglycemia is associated with more frequent inflammation, infections, clinico-metabolic complications, prolonged length of hospital stay and increased mortality. Van der Berghe et al observed adverse effects on the nervous, respiratory, immune and renal systems [1]. But hypoglycemia is equally dangerous. The adverse consequences of hypoglycemia include those on circulatory, autonomic, and central nervous systems. There is no doubt that tight glycemic control is essential for rapid wound healing and good outcome. Intensive glycemic controls in line with established insulin protocols no doubt minimizes insulin resistance and hyperglycemia. The controversy continues to exist in the context of establishment of target blood glucose levels and protocolization of the generalized indications for intensive insulin therapy initiation [2].
Unfortunately, perioperative hyperglycemia has been associated with the greater risk of postoperative morbidity and mortality [3-12]. Several studies have concluded that adequate glycemic control without excessive hyper- or hypo-glycemia has been associated with an improved surgical outcome [13-20]. All these studies have shown strong correlation between postoperative hyperglycemia and poor surgical outcome in both cardiac and non-cardiac surgery, though majority of surgeries are on cardiac surgery patients. This review article addresses the ever debatable issue on perioperative glycemic control in diabetic patients and focuses on recent advances and updates in the optimization of perioperative blood glucose levels, merits and demerits of various perioperative regimens, with recent studies on the effects of optimal perioperative glucose control on morbidly obese patients undergoing bariatric surgery, and recent studies on preoperative HbA1c and the associated risk of postoperative compilcations in patients undergoing oncosurgery.
Insulin synthesis and secretionInsulin is produced in the beta cells of pancreatic islets. Synthesized as a single chain 86 amino acid polypeptide preproinsulin, it undergoes subsequent proteolytic processing to give rise to proinsulin. Proinsulin undergoes cleavage to form c-peptide and the A (21 amino acids) and B (30 amino acids) chains of insulin which are connected by disulphide bonds. Both, Cpeptide and insulin molecules are stored together and cosecreted from secretory granules in the beta cells. The key regulator of insulin secretion by the beta cells is Glucose, although amino acids, ketones, gastrointestinal peptides and neurotransmitters also influence secretion of insulin.
Diabetes- A pro-inflammatory stateRecently, Jagannathan and colleagues concluded through a study that altered B cell function in diabetic patients was responsible for the pro-inflammatory state in diabetes patients [21]. The altered Toll like receptor function of B cells led to both up regulated expression of Interleukin 8 and down regulated expression of Interleukin 10. Either of the two led to enhanced inflammatory response in these patients [21].
Clinical Complications of DiabetesDiabetes almost affects each and every organ or system leading to both microvascular and macrovascular complications. In the cardiovascular system, it leads to premature atheroma formation, thus magnifying the risk of coronary artery disease in diabetic patients. Patients with underlying component of diabetic autonomic neuropathy (DAN) show an enhanced risk of developing silent Myocardial Ischemia and infarction. Patients suffering from this ‘Cardiacdysautonomia’, also develop sudden hypotension post induction with negligible tachycardic and hypertensive response to intubation. There is also increased incidence of hypertension and its sequale in these patients.
Central Nervous system manifestations of diabetes include Peripheral neuropathy and Autonomic Nervous System Dysfunction. Peripheral Neuropathy leads to increased risk of nerve injury and ischemia, which mandates care at positioning and transport and cautious use of nerve blocks during regional anesthesia. Also, increased incidence of cerebrovascular attacks has been observed in diabetics.
Respiratory system bears the major brunt of diabetic involvement. There is markedly depressed ventilator response to both hypoxemia and hypercarbia, as a result there is enhanced susceptibility to the ventilatory depressant effect of drugs. Increased risk of respiratory tract infections explains the increasing risk of postoperative pneumonia seen in diabetic patients, especially post thoracic and upper abdominal surgery. Lung volumes, forced vital capacity and forced expiratory volume are reduced due to glycosylation of tissue proteins in connective tissues. Oxygen transport is also affected by diabetes as glucose covalently binds to hemoglobin molecules and alter the allosteric interactions between beta chains.
Diabetic patients present with difficult airway (from the perspective of the dealing anaesthesiologist) due to restricted neck movements as a result of nonenzymatic glycosylation of proteins and abnormal cross linkage of collagen. This leads to difficult laryngoscopy and intubation. The degree of involvement of joints can be assessed by ‘The Prayer sign’ and ‘Palm Print sign’.
There is increased risk of acute renal shutdown in these patients, especially in the perioperative period, attributable factors include intrinsic renal disease, hemodynamic impairment and urosepsis. Urinary tract infect remains most common postoperative complication in these patients.
Increased risk of vitreous hemorrhage exists in these patients, especially while performing laryngoscopy and intubation. Acute complications include Diabetic ketoacidosis, Nonketotichyperglycemic coma and Hypoglycemia. There is also increased risk of hypothermia and wound infections in the postoperative period.
Various Regimens for the Perioperative Control of Blood SugarIn 1979, British journal of anaesthesia published an article by Alberti and Thomas in which they introduced i.v. infusion of a premixed bag of glucose 105- Insulin 10 units- Potassium 10 meq, for the perioperative management of diabetic patients to be infused @ 100-125 ml/hour [22]. If the infusion stops, the patient’s ECF will contain no effective insulin within 30 minutes, therefore one must ensure that this infusion is continuous. It is typically used in Type 1 diabetes and insulin controlled type 2 diabetes. It includes stabilizing the blood sugar levels 2-3 days prior to surgery and then shifting to short acting insulin on day before surgery. Dextrose insulin potassium drip@ 100 ml/hour (10,10,10) is started after omitting the morning dose of insulin, on the morning of surgery. The advantages of Alberti Regimen, include its simplicity and inherent safety. The disadvantages include fluid overload, hyponatremia, and hyperglycemia. In an intensive study, Husband et al concluded that this Alberti regimen showed efficacy in 82% of diabetic patients in the perioperative period and that optimal results depended on careful monitoring with appropriate alteration of strategy [23].
It is also suggested that in preoperative starvation, provision of insulin with sufficient carbohydrates @ 180 gm/day is necessary to mitigate uncontrolled catabolism. It is also necessary to understand the normal endocrine and metabolic response to starvation and surgery and also to understand that how this may be modified in the diabetic patient. The duration of preoperative starvation and postoperative starvation has to be kept in mind.
Several factors are needed to increase insulin requirements and these include severity of surgery, especially cardiac surgery, presence of underlying infection, administration of glucocorticoids and catecholamines. It is also recommended that if the diabetic patient was >50% heavier than their ideal body weight, then the dose of insulin should be increased twofolds. Risk of hyponatremia from prolonged infusion of glucose is now well recognized. So sodium chloride 0.9% is an acceptable fluid for use in diabetic patients. Today, even after 37 years of practice of Alberti regimen, the Alberti regimen still has the unlimited radiance of a precious diamond [24].
Tight-control RegimenWith the goal to maintain Blood Sugar level in the range of 79-120 mg%, it is especially indicated in pregnant patients, patients undergoing Cardiopulmonary bypass, neurological surgeries and those requiring postoperative care. 5% Dextrose infusion is started @50 ml/hour, with an infusion of regular insulin (50U insulin in 250 ml of 0.9% insulin) piggyback to it. Positive outcomes were observed after instituting this method in terms of improved wound healing and decreased incidence of wound infection, improved neurological outcome and weaning from cardiopulmonary bypass. But factors like difficulty in maintaining meticulous frequent monitoring, especially in ward setting and higher chances of hypoglycaemia have limited its use in the present clinical scenario.
Stoelting’s regimen/Intraoperative Insulin RegimenThis regimen is used during intraoperative management of patients, which includes starting an insulin infusion @ of 0.02 U insulin/kg /hour, along with 5% Dextrose normal saline with 20 meq KCl @ 100-150 ml/hour. The aim is to maintain Blood Sugar levels between 120-180 mg/dl. It consists of 4 algorithms with Algorithm 1 starting for most of the patients initially and algorithm 2 starting for patients receiving glucocorticoids, vasopressors, ones with post coronary artery bypass grafting (CABG) status. Patient is moved up the algorithm if blood glucose level is outside the goal range for 2 hours or the blood glucose (BG) level does not change by atleast 60mg/dl within an hr. Patient is moved down the algorithm when two consecutive blood sugar levels <70 mg/dl or if BG level decreases by more than 100 mg/dl within an hour. Regular BG monitoring is done for every hour until within the goal range for four hours, subsequently every 2 hours for next 4 hours and ultimately every 4 hourly [25]. Stoelting’s regimen is summarized in Table 1 below
Regional Anesthesia and Optimization of Perioperative Blood Glucose LevelsRecently Nair et al. in an interesting study involving 2440 patients scheduled for non-cardiac surgery, observed that a higher intra-operative blood glucose levels were associated with higher postoperative blood glucose levels [26]. They concluded that intra-operative hyperglycemia predisposed towards increase in the odds for postoperative hyperglycemia. Nair et al also added that optimization of intraoperative by starting insulin infusion when the glucose levels exceeded 140 mg/dl led to lower postoperative glucose levels and minimized the incidence of postoperative hyperglycemia [26].
Blood Glucose Optimization in Critically ill PatientsVan den Berghe et al. conducted the first trial regarding the blood glucose optimization and concluded that tight glucose control with target Blood Glucose levels of less than 110 mg/dl had tremendous benefit on the outcome of critically ill patients [27]. However, this conclusion was refuted by further studies by NICE-SUGAR (Normoglycemia in Intensive Care evaluation and Survival using Glucose Algorithm Regulation) group, which concluded that intensive glucose control led to moderate and severe hypoglycaemia, both of which were associated with an increased risk of death [28].
OutcomesThe data from several studies proved that perioperative hyperglycemia predisposes to postsurgical complications in cardiac surgery, vascular surgery and colorectal surgery [1-10]. Nair et al concluded that a raised mean intra-operative blood glucose concentration, was concomitantly associated with raised mean postoperative blood glucose concentration in surgery patients [19]. Recently, Abdelmalak et al. observed that the use of steroids intraoperative had significant positive effects on the blood glucose concentration [29]. Additionally, age and BMI also had significant positive effects on raised postoperative glucose levels. Increased odds for postoperative hyperglycemia were noted in association with intraoperative hyperglycemia (more than 180 mg/dl), though this was also noted when intraoperative glucose levels were higher than 140 mg/dl.
It is a well-known fact that raised blood glucose concentrations during surgery are frequently managed by initiating insulin infusion. This has been established by ADA (American Diabetes Association), Subramaniam et al. and Jacobi et al. [30-32]. Obviously things are not clear as to when initiate insulin infusion. There is no doubt about a variable practice among anesthesiologist due to the concern and fear of hypoglycaemia during anaesthesia and unclear target for glucose management. The study by Nair et al. showed that initiation of insulin at a glucose level of 140 mg/dl did result in lower postoperative glucose levels and minimal incidence of postoperative hyperglycemia. The additional benefit of initiating insulin infusion at an early stage at the glucose level of 140 mg/dl did not increase the incidence of hypoglycaemia [26]. The association between postoperative glucose levels and postoperative outcome has already been well established by Zerr et al. Vriesendrop et al. Ramos et al. McConnell et al. and Ata et al. [3,5,9,10,12].
Recently, de Vries et al. conducted a meta-analysis of databases from PubMed, Embase, CENTRAL, CINAHL and WHO from 1st January 1990 to 1st August 2015 where inclusion criteria were randomized control trials comparing intensive with conventional glucose control protocols. They concluded that stricter and lower blood glucose levels of <150 mg/dl(8.3 mmol/L) using an intensive protocol in the perioperative period did result in reduction of surgical site infection with an inherent risk of hypoglycemic events but without a significant increase in adverse events [33].
Also, as regards to the glycemic outcome, recently Arnold et al conducted a study for evaluation of glycemic outcome at 3 years after implementation of perioperative glycemic control algorithm. Patients with known Diabetes mellitus and scheduled to undergo surgery were included. In a study involving 848 patients scheduled to undergo surgery and with known diabetes mellitus (260 in control group and 588 in the interventional group) (blood glucose levels >180 mg/dl), a reduction in perioperative blood glucose levels and rate of hyperglycemia were observed [34]. Recently Dhataraiya et al. [35] observed that glycaemic targets for the perioperative period should be ensured to maintain blood glucose levels between 108- 180 mg/dl (6.0-10.0 mmol/L). They also recommended that early involvement and consultation with a diabetes expert was essential if the HbA1c value is more than 8.5%.
Yoo et al. in a very recent retrospective observational study explored association between perioperative hyperglycemia or glucose variability and postoperative AKI (Acute kidney injury) after liver transplantation. They concluded that increased perioperative glucose variability and not hyperglycemia was independently associated with increased risk of postoperative AKI in liver transplant recipients [36].
With special reference to cataract surgery under regional anesthesia in diabetic patients and the perioperative glycemic control in four public hospitals in Singapore, recently Woo et al. conducted a questionnaire survey of 76 doctors from ophthalmology department and 53 doctors from anaesthesia department. A blood glucose level of ≥17 mmol/L prompted 86.0-93.8% of respondents to adopt a treat and defer strategy, and a level of ≥ 23 mmol/L prompted 86.0-96.9% of respondents to cancel the cataract surgery. The respondents were consistently more concerned about perioperative hyperglycemia than intraoperative hypoglycemia [37].
Preoperative HbA1c and risk of postoperative complications in cancer patientsIn another interesting study of 300 patients of gynaecological cancer (34 diabetic and 266 non diabetic) Iavazzo et al., concluded that preoperative measurement of HbA1c levels might identify patients (diabetic and nondiabetic women) at higher risk of postoperative complications and could be used as a trigger for modification of perioperative management of such patients with gynaecological cancer [38].
Stress hyperglycemia and peioperative glucose controlWith specific reference to perioperative stress hyperglycemia, recently Tanaka et al. showed that prognosis of perioperative patients was more likely to be greatly improved if we can control stress hyperglycemia [39]. One should not forget that volatile anesthetics inhibit insulin secretion after glucose load and affect glucose tolerance. They suggested that it was necessary to perform the glycemic control of patients who fell into the stress hyperglycemia depending upon individual patient.
Intraoperative real time electronic remindersWith specific focus on intraoperative blood glucose management, Nair et al concluded that real time electronic reminders improved intraoperative compliance to institutional glucose management protocols, though glycemia parameters did not improve when there was greater compliance to the protocols [40].
Optimization of perioperative glucose levels in morbidly obese patients undergoing bariatric surgeryIn a retrospective analysis study of 155 morbidly obese patients, who underwent laparoscopic gastric bypass (RYGB) or sleeve gastrectomy (LSG) procedures, Zaman et al concluded that bariatric surgery led to significant reduction of type II diabetes and a significant improvement in glucose tolerance in perioperative period [41].
It is suggested that an attempt must be made to explore the effects of type and severity of Diabetes and HbA1C levels on intraoperative and postoperative blood glucose concentrations. Nair et al. and Abdelmalak et al. observed that the use of steroids intraoperatively was associated with raised perioperative blood glucose concentrations [26,29].
Webster et al in a recent editorial suggest that tight control regime was not of much benefit in the critically ill patients, but at the same time, not much has been explored as regards the perioperative diabetic patient [42]. However, Webster et al. suggest that an investigation of the use of carbohydrate restriction to control circulating glucose concentrations in the critically ill as well as the perioperative patients is warranted in future (42). Another part of consideration is that whether the glucose measurement was done using the point of care glucometer?? It has been noted by Akhtar et al. and Kadoi et al. that the guidelines for intraoperative glucose management remain nonstandard and poorly followed by anesthesiologists [14,17].
ConclusionTaken together, an early and adequate management of raised intraoperative glucose levels, results in lower starting blood glucose concentrations for postoperative glucose management. The age and BMI also have significant positive effects on raised postoperative glucose levels. Also, initiation of insulin infusion early, at 140 mg/dl blood glucose concentration does not increase the incidence of hypoglycaemia. It is a well-established fact that various perioperative regimens have been used for diabetic patients, optimal results depend on careful monitoring with appropriate alteration of therapy. Let us remember that each diabetic patient is different from the other, both phenotypically and genotypically. Hence, the take home message is that perioperative regime has to be flexible and common sense must be applied at every step during the perioperative management of a diabetic patient.
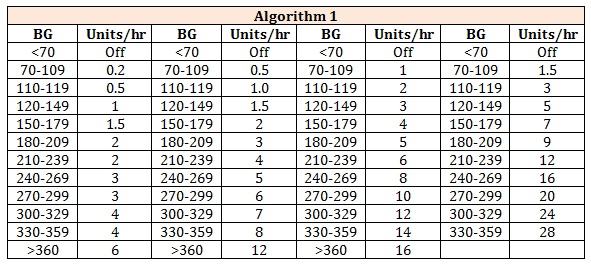
Table 1: Stoelting’s regimen is summarized.
Chat with us on WhatsApp