
Citation: Adegoke OO, et al. Microsatellite Instability Statuses and Clinicopathological Characteristics of Colorectal Carcinomas in a Sub-Saharan African Population. Gastroenterol Hepatol Int J 2017, 2(2): 000119.
*Corresponding author: Department of Anatomic Pathology, Bowen University, Iwo, Nigeria, Email: oluwafadekemiadegoke@gmail.com
Context: Colorectal carcinomas (CRC) in Africans tend to occur at an earlier age and have unique clinicopathologic as well as putatively distinctive molecular characteristics. We recently undertook a study of the microsatellite instability (MSI) analysis of a cohort of colectomies in our practice to examine this issue further.
Methods: Fifty-five (55) colectomies done for colorectal carcinoma were evaluated. The pathological features of the tumours were recorded. Microsatellite Instability (MSI) analysis was carried out using immunohistochemistry for the DNA mismatch repair proteins, MLH1 and MSH2.
Results: The average age was 49.2 years and the highest number of cases was seen in the 6th decade of life. Nineteen (34.5%) of the tumours in this study were found to be MSI-positive. Fourteen (73.7%) of the MSI tumours were found in patients below 50 years of age. There was a significant statistical association between age and MSI-status (p=0.004). Eleven (58%) out of the MSI tumours were located in the right colon while 8 (42%) were located in the left. On the other hand, 24 (77%) of the tumours with a normal MMR immuno profile were located on the left and 10 (23%) on the right. There was a statistically significant association between the site of tumour and microsatellite instability status (p=0.012). Adenocarcinoma was the commonest histological type making up 65.5% of all the tumours. This is closely followed by mucinous carcinomas (32.7%) and signet ring carcinomas (1.8%). Eleven of the 18 mucinous carcinomas were found in patients under 50 years of age. Nine (50%) of the mucinous carcinomas were MSI-positive although this was not statistically significant. There was no case of generalised polyposis found in this study.
Conclusion: Our findings is consonant with a lower average age (49.2 years) for colorectal carcinomas in similar settings as ours and contrasts with high-incidence CRC in Caucasians in who the reported average age is consistently above 60 years. A significant number of tumours where deficient in mismatch repair proteins and these tumours tended to be right-sided, mucinous and to occur at an earlier age. These findings signal the need for further investigation of alternative pathways for the aetiology and pathogenesis of colorectal carcinomas in our region.
Colorectal carcinomas are common in Caucasians where an established pathway of carcinogenesis i.e. the adenoma-carcinoma sequence has long been described.
Colorectal carcinoma has a much lower incidence in most parts of Africa, its clinicopathological associations are not and also appears to have unique molecular and clinicopathologic characteristics. For example, there is little association with the typical premalignant conditions such as polyps [1-3].
In addition, workers most especially across Africa and the Middle East [2-6], have pointed to the apparent differences in the biological behaviour of colorectal carcinoma in these low-incidence type: the patients tend to be younger and tend to present with tumours that tend to be poorly differentiated with more cases being of the mucinous and signet-ring histological types.
Although in its early days, emerging data from molecular biological studies on low-incidence CRCs seem to lend credence to the notion of 2 epidemiological types of CRCs. For example, Soliman et al, working in Egypt, reported that patients tend to present with tumours in which micro satellite instability (MSI) was commoner while mutations in KRAS were lower than is reported for Westerners [7]. On the converse, Western CRCs tend to observe Adenoma-Carcinoma sequence, thus mostly have molecular features in keeping with the chromosomal instability pathway such as mutations in the KRAS oncogene, while there are fewer cases of microsatellite instability when compared with Africans [8]. A recent Nigerian study done in Lagos, Nigeria found KRAS mutations occurred in about 21% of tumours studied [9]. This is about half the rates in Caucasians and further supports the notion that the molecular pathogenesis of these carcinomas may be different.
Microsatellite instability accounts for up to 15% of sporadic colorectal carcinoma [10]. It arises from failure of repair of DNA mismatches immediately after DNA replication in microsatellites [9,10]. Microsatellites are repetitive sequences made up of mono-nucleotide, dinucleotide or higher order nucleotide repeats [11]. Mutations in DNA mismatch repair (MMR) genes responsible for the recognition step lead to accumulation of errors in DNA resulting in microsatellite instability [12]. Although it was first described in colorectal carcinomas it has subsequently been observed in diverse tumour types including gastric, endometrial and ovarian tumours [9-12]. Lynch syndrome is due to an inherited germline mutation in a single allele of the MMR gene, a second somatic hit on the other allele is required for the defect in MMR to be evident [10]. These patients usually develop multiple colon tumours between 20 and 30 years of age and may also have extra-colonic tumours [10]. Tumours with MSI usually lack mutations in KRAS and P53 but genes such as TGFBRII, EGFR and BAX which contain simple repeats are usually mutated [11]. Colorectal carcinomas that are microsatellite unstable have unique phenotypic features which include, a tendency to be located in the right colon, a medullary carcinoma phenotype, the presence of a mucinous or signet ring component, the presence of tumour infiltration and peritumoral lymphocytes, a Crohn-like inflammatory infiltrate and a pushing tumour border [10-12]. Sporadic tumours that are microsatellite unstable are thought to have a more favourable prognosis [13], however MSI has also been associated with a lack of response to the fluorouracil-based adjuvant chemotherapy regimen [14], a widely used protocol. Some studies have shown MSI among colonic epithelial cells in inflammatory bowel disease suggesting colorectal carcinogenesis may follow this pathway in these patients.
This study aimed to look at the degree and nature of microsatellite instability in colorectal carcinomas in Nigerians.
MethodsWe carried out a retrospective study of consecutive cases of colorectal carcinomas for which colectomy was done. The biodata and other demographic information of the patients were extracted from the records. The pathological features of the tumour including tumour site, gross growth pattern, histological type, TNM stage and incidental pathological lesions were recorded. Microsatellite instability analysis was carried out using immunohistochemistry for the DNA mismatch repair proteins, MLH1 and MSH2. MLH1 and MSH2 are mutated in a vast majority of microsatellite unstable tumours and using immunohistochemistry to detect their loss has shown a specificity of 100% and sensitivity of 92.3% when compared to molecular testing [15]. Immunohistochemistry was carried out on the formalin fixed paraffin embedded (FFPE) tissue sections. Tumour tissue with loss of staining relative to adjacent normal epithelium or intratumoral lymphoid or stromal cells where assigned negative staining and deemed to have loss of expression of the DNA mismatch protein. Tumours which showed loss of staining in both the tumour and adjoining normal tissue were excluded from the study.
ResultsOf the 55 cases reviewed, the average age was 49.2 years and the highest number of cases was seen in the 6th decade of life. Nineteen (34.5%) of the tumours in this study were found to be MSI-positive. Fourteen (73.7%) of the MSI tumours were found in patients less than 50 years of age. There was a significant statistical association between age and MSI-status (p=0.004). The Male to Female ratio in this study was 1.12.
Left sided tumours made up 60% of the colorectal carcinomas in this study while the remaining 40% were located on the Right side. Eleven (58%) out of the MSI tumours were located in the right colon while 8 (42%) were located in the left. On the other hand, 24 (77%) of the tumours with a normal MMR immunoprofile were located on the left and 10 (23%) on the right. There was a statistically significant association between the site of tumour and microsatellite status (p=0.012).
In this study, the exophytic growth pattern was the commonest accounting for 60% of the cases. This was followed by the endophytic/ulcerative growth pattern (24%) and then the annular growth pattern, which was the least common. Most of the MSI-positive tumours in our study had an exophytic growth pattern (73.7%), suggesting a possible association of microsatellite instability with this growth pattern.
Among our cases, adenocarcinoma was the commonest histological type making up 65.5% of all the tumours. This is closely followed by mucinous carcinomas (32.7%) and signet ring carcinomas (1.8%). there were slightly more females (20, 54%) than males (17, 46%) with adenocarcinomas, although this difference was not statistically significant and it was the reverse for mucinous carcinomas where there were 12 males and 5 females. Eleven of the 18 mucinous carcinomas were found in patients under 50 years of age. Nine (50%) of the mucinous carcinomas had MSI although this was not statistically significant.
There was no case of generalised polyposis found in this study, however one case had an adenomatous polyp about 8 cm from the tumour. This case was that of a 74- year-old male who had signet ring carcinoma. The tumour in this patient was microsatellite stable.
DiscussionColorectal carcinomas are now known to be heterogenous diseases arising through various molecular pathways. In recent times, much attention has focused on microsatellite instability as an important molecular pathway of colorectal carcinomas in areas where there is a low incidence and the diseases do not seem to follow the well-recognised adenoma-carcinoma sequence. Indeed, studies have reported the low incidence of adenomatous polyps among Africans [3,16]. This was also reflected in this study where there was a notable lack of premalignant conditions such as inflammatory bowel disease and polyposis suggesting that this may not be an important pathway to tumour formation in our own patients.
Our study examined the microsatellite instability status in a cohort of Nigerian patients where the incidence of colorectal carcinoma although significant is not as high as in Caucasians.
A significant number of tumours in our study were microsatellite unstable (34.5%). This number is much higher than what has been reported in Western Countries and Asia where the values are much lower [8]. A high value of 37% was reported in Egypt, all pointing to the presumed importance of microsatellite instability in colorectal carcinoma in Africans and people of African descent. Studies from Egypt in Africa, Nigeria and amongst African-Americans have also reported a high rate of mismatch repair protein deficiency in colorectal tumours [7,17,18].
Data from this study shows a lower average age (49.2 years) for colorectal carcinomas in this environment suggesting an earlier onset when compared to Caucasians where the average age reported is consistently above 60 years [19,20]. This finding is similar to studies from other sites in Nigeria and other low incidence areas [21,22]. The microsatellite unstable tumours also tended to occur at an earlier age, similar to what Duduyemi reported in Ibadan, Nigera [17].
Although adenocarcinomas were the predominant histological type, 33% of the tumours were mucinous carcinoma and half of these mucinous tumours were microsatellite unstable. On the face of it, this raises a spectre of a prominent role for MSI in the pathogenesis of mucinous carcinomas in our patients. Local studies including ours have suggested that a significant number of colorectal carcinomas in young Nigerians are of the mucinous phenotype and a right-sided topography [21]. It is obvious that colorectal carcinomas in Nigerians and other Africans have unique clinicopathological characteristics, much has been said about this in many studies. It is now also apparent that these tumours may have unique molecular characteristics, which will have possibly great impact on their prognosis and therapeutics. Microsatellite-unstable tumours, for instance, have been found to respond poorly to 5-flourouracil based adjuvant chemotherapy [11,23] thus the MSI phenotype may be predictive of the response to neo-adjuvant therapy using this agent. Paradoxically, tumours with the MSI phenotype have also been found to have a better prognosis [13,14].
Microsatellite instability in colorectal carcinomas has great impact on the clinical course of disease. As said earlier, these tumours tend to occur in the young, have a better prognosis and a peculiar response to adjuvant chemotherapy. Testing cases with a histomorphology consistent with MSI in routine practice will help identify patients who have this mutation and manage them appropriately. It will also enable physicians to carry out further testing and perhaps even pick some people who have a hereditary cancer syndrome. The knowledge gained from determining the MSI phenotype of colorectal carcinomas will also go a long way in predicting response to chemotheurapeutic agents, for example it is thought that a combination of drugs without excessive immunotoxicity may work better for patients with MSIassociated CRC while the traditional 5-FU based therapy is not very useful [23].
LimitationsImmunohistochemistry was used in this study work optimally with appropriately fixed tissues. Some colorectal carcinomas with defective DNA mismatch protein may show normal expression of the MMR proteins. Possible reasons include an unevaluated MMR gene such as MSH3 where only MLH1 and MSH2 have been evaluated or that one of the genes is expressed but not functioning properly. Studies have shown that not all pathogenic mutations lead to loss of protein expression using IHC, this is particularly true for MLH1 where up to third of cases are due to missense mutations that may result in mutant proteins that are catalytically inactive but antigenically intact [24]. Immunhistochemical staining has also been associated with a varying staining pattern which may make interpretation difficult, some of these include focal staining with weak intensity and lack of internal control in negatively stained tumours [24]. Some of these issues were encountered in this study. MSI testing using IHCs is also limited by the antibody panel used, for example using only MLH1 and MSH2 as was done in this study will pick out tumours with loss of MLH1/PMS2 or MSH2/MSH6 whereas mutations of PSM2 or MSH6 often cause isolated loss of PMS2 or MSH6 only and so would be missed [24,25]. Also, it is possible for MMR deficiency to be caused by mutations in MMR genes not tested by IHC or in as yet unidentified MMR gene whereas MSI testing will shoe positive results in the presence of a mutation that disrupts the normal MMR function, whether the mutation resides in a known gene or in an uncharacterized MMR gene [25]. Although there are limitations in the use of immunohistochemistry for MMR deficiency, some advantages also exist. It is cheap, widely available and can easily be done as part of routine clinical laboratory practice. IHC for DNA MMR proteins also allows for identifying the mutated gene thus enabling more efficient mutation analysis [24].
ConclusionMicrosatellite Instability accounted for a significant number of colorectal carcinomas in our cohort and these tumours exhibited distinctive clinicopathological characteristics. Although direct molecular studies, and in larger cohorts, need to be done to validate our findings, it is obvious that tumours in our patients, as in other lowincidence areas, have unique clinicopathological characteristics. Doubtless, the role of MSI in African CRC needs a closer and wider examination.
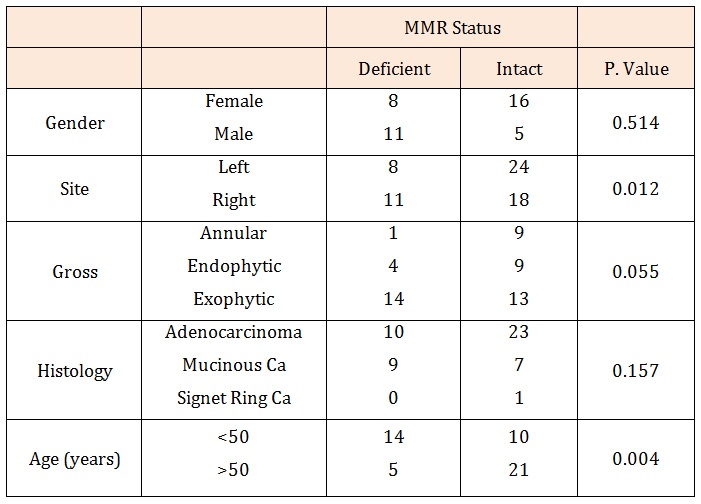
Table 1: Showing the association of clinicopathological characteristics with MMR status and statistical significance.
Chat with us on WhatsApp