
Citation: Ooi M and Thomson A. An Observed Geographic and Temporal Cluster of Newly Diagnosed Menetrier Disease Cases. Gastroenterol Hepatol Int J 2017, 2(2): 000123.
*Corresponding author: Dr Marie Ooi, Gastroenterology and Hepatology Unit, PO Box 11, Woden ACT 2606, Level 2, Building 1, The Canberra Hospital, Yamba Drive ACT 2606, Australia. Contact: 0430669940, Email: marieooi_al@yahoo.com
Menetrier’s disease (MD) is a rare idiopathic disease, characterized by giant gastric hypertrophic folds, hypochlorhydria and hypoalbuminemia secondary to protein loss through the gastric mucosa. MD was first described in 1888 and since then, there have been very few case reports from various countries. Herein we describe an unusual spatio-temporal aggregation of 3 patients diagnosed with MD. Despite detailed analysis of the characteristics and similarities of the cases, no discernible links or causative factors were identified, most likely due to the very small sample size in a rare disease entity. However, these cases may serve as a starting point for further epidemiological studies to characterise this rare and intriguing entity.
Keywords: Menetrier’s Disease; Hypertrophic Gastropathy; Protein Losing Gastropathy; Transforming growth factoralpha; Epidermal growth factor receptor; Cetuximab
Menetrier’s disease (MD), also known as hypoproteinemic hypertrophic gastropathy, is a rare, acquired premalignant disorder of the stomach which is characterized by giant hypertrophicfolds, mainly involving the fund us and the greater curvature of the body of the stomach, typically sparing the antrum [1]. Histologically, it is characterized by elongation and tortuosity of the epithelial pits and increasing inflammatory cells in the pit region with cystic dilatations of the body mucosa [2]. Clinically, there is excess mucus secretion, hypochlorhydria, protein losing gastropathy and hypoalbuminaemia [3,4]. There is a bimodal age distribution: The first peak is in childhood with a mean age of 5.5years and the second peak occurs in adulthood with an average age of 55 years [3]. In both groups there is a distinct male predominance and it is more common in Caucasians. There are significant differences between adult and pediatric cases in terms of presentation, pathogenesis and prognosis [3]. Two-thirds of the adult onset cases have an unfavorable prognosis and tend to progress over time, requiring either a partial or total gastrectomy to control symptoms and to prevent gastric cancer. In contrast, the childhood form has a self-limiting clinical course, often requiring only supportive management with a high protein, low salt diet. Only 10% of children have a lifetime risk of requiring surgical intervention. The etiology of this disorder remains unclear, although several exogenous agents such as chemical irritants and toxins have been implicated to explain the gastric mucosal hyperplasia. In the pediatric MD, there are case reports associating MD with viral infections such as cytomegalovirus (CMV), Helicobacter pylori, autoimmune disease and allergic processes [5-10]. Recent studies implicate an over expression of transforming growth factor alpha with increased signaling of the epidermal growth factor receptor as the underlying pathogenesis in the adult form making anti TGF- α agent, such as cetuximab, potential treatment options [11-13].In this report, we describe a small cluster of 3 cases of adult onset Menetrier’s disease occurring in a demographically isolated area of inland Australia in the same year. All 3 cases underwent a total gastrectomy with histological confirmation of the diagnosis. One patient received a trial of cetuximab prior to surgical resection and another patient had underlying ulcerative colitis (UC), which has been associated with MD.
Case 1A 43-year-old Caucasian panel beater with a 14-year history of pan-ulcerative colitis which had led to total colectomy and permanent ileostomy one year after diagnosis, mitochondrialmyositis, bronchiectasis and asthma presented with a 1-month history of malaise, chronicvomiting, epigastric pain, 10 kilogram weight loss and bilateral peripheral edema. Hismedications were amitriptyline, oxycodone, fluticasone inhaler, pantoprazole, itraconazole Laboratory studies revealed microcytic anemic with a hemoglobin of 94 (normal range 135-180g/L), elevated serum creatinine of 105 (normal range 40-90umol/L), albumin 29 g/L(normal range 33- 50g/L) and C reactive protein 16.2 mg/dL (normal range < 5mg/dL). Anabdominal computed tomography (CT) revealed marked diffuse hypertrophy of the entire stomach with a gastric wall thickness of 17mm in the body and antrum (refer to figure 1). Gastroscopy revealed diffuse hypertrophy of gastric rugae with diffuse polypoid lesions. Histology from the gastric biopsies demonstrated marked foveolarhyperplasia associated with cystic gland dilatation and chronic inflammation with eosinophilia consistent with MD (refer to figure 2). Immunostaining for Helicobacter pylori and cytomegalovirus (CMV) were negative and there was no evidence of dysplasia or carcinoma. He was treated with prednisolone 40mg orally daily for 4 months but due to persistent symptoms, a total gastrectomy was performed. Histology confirmed the diagnosis of Menetrier’s disease. His symptoms resolved and he has not developed a recurrence of symptoms for 4 years.
Case 2A 32-year-old draughtsman presented with a five-week history of non-bloody diarrhea (4-5bowel actions per day) on a background of a six-month history of fatigue, anorexia and weight loss. Co-morbidities included asthma, eczema and migraine. Medications were salbutamol, seretide, ergotamine, and a topical steroid. He was a lifelong non-smoker and drank 1-2glasses of wine a week. There was a family history of bowel cancer (father).On physical examination, he was comfortable but was cachectic with prominent ribs, thin extremities and wasted musculature. The conjunctivae were pale and there was pedaledema to the ankles. His abdomen was soft and non-tender. There was a microcyticanemia with hemoglobin of 86g/dL. The platelet count was 553 x 109 g/L (normal range150-400x109 g/L) His electrolyte is within normal range and his serum protein and albumin were normal at 69g/L and 44g/L respectively. Stool analysis including bacterial culture and Clostridium difficile toxins were all negative. Urinalysis revealed a specific gravity of 1.027, pH5.5 with a trace of albumin. Abdominal CT demonstrated marked thickening of the greater curvature and lesser curvature of the stomach (Figure 3). The remaining organs appeared normal and there was no lymphadenopathy. Gastroscopy revealed varioliform gastritis with thickened gastric folds associated with diffuse polypoid lesions with sparing of the greater curvature. There was a focal area of nodular ulceration, the biopsies of which revealed invasive adenocarcinoma. Helicobacter organisms were not seen. Total gastrectomy revealed features of diffuse polypoid lesions with hypertrophic gastropathy and an area of central depression with large polypoid edges located at the mid body of the stomach (Figure 4). On histology, the adenocarcinoma was confined to the lamina propria that appeared to have arisen from the hypertrophic gastropathy.
Case 3A 28 year old male previously well public servant who worked in the department of defence presented in 0ctober 2008 with a 4 month history of progressive fatigue, weight loss, anorexia and bilateral pedal edema. His CT abdomen revealed marked diffuse hypertrophy of the entire stomach with a gastric wall thickness of 20mm at the gastric body. A gastroscopy revealed sheets of polypoid cystic lesions throughout his stomach sparing segments of the greater curve. Biopsy showed similar features as in Case 1 with foveolarhyperplasia, cystic dilatation of glands with glandular atrophy and chronic inflammation. There was extensive goblet cell metaplasia. No dysplasia or carcinoma was noted histologically. The diagnosis of Menetrier’s disease was made and molecular testing of the biopsies for epidermal growth factor receptor (EGFR) was negative. A trial of cetuximab was undertaken without improvement. A total gastrectomy was thus undertaken and the gross specimen revealed extensive polypoid lesions throughout the stomach (refer to Image 5). The microscopic findings showed elongated foveolar epithelium with foveolar mucous cell hyperplasia and cysticdilation of the foveolar glands. Additionally, there were acute inflammatory lesions with neutrophil infiltration confined to the muscularis propria. The pathologic diagnosis was Menetrier’s disease. Post resection his symptoms resolved and he regained his weight and has remainedasymptomatic for four years.
DiscussionMenetrier’s disease is a rare but important consideration in the differential diagnosis of gastricpolyposis in patients with hypoalbuminemia, hypochlorhydria, and localized findings of gastricpolyps [1]. These 3 cases occurred in consecutive months in a small regional city of Australia. To the best of our knowledge, this is the first temporal and geographical cluster of Menetrier’s disease reported in the literature. The detection of such a cluster raises the possibility of a common environmental agent. Canberra has a population of about 350 000 and is surrounded by hundreds of kilometers of farmland in every direction. It is Australia’s capital city and as such it houses most of Australia’s foreign embassies and high commissions. There is also a large population of Australian diplomats and military personnel, who partake in consider able international travel. None of our patients to our knowledge had contact with each other and none were employees neither of any overseas countries nor of the Australian Foreign Service. There was a well-known kangaroo cull in Canberra, around the time these 3 cases occurred. However, the significance of this event is questionable and is most likely a co-incidence. None of our patients worked on a farm and there are no abattoirs or live stock markets in Canberra. A zoonosis thus seems highly unlikely as a potential cause. These reported cases were similar to other cases in the literature. The association of MD with ulcerative colitis is uncommon but well described with at least nine previously reportedcases [14]. There is evidence, implicating overstimulation of EGF alpha receptors to the pathogenesis of MD [11-13]. The lack of response to cetuximab in one of our cases indicates that there may potentially be other factors involved in its occurrence and perpetuation.
ConclusionWe hereby described a small series of a rare disease entity without any apparent epidemiological basis. Given the very small sample size, we were unable to draw any conclusion as to the etiology or pathological relationship among the 3 cases. However, this case series serve as a starting point for further studies to characterize and to determine whether there are environmental factors at play for this peculiar.
SummaryThis article illustrates a series of unusual spatiotemporal aggregation of Menetrier’s disease (MD) that is an extremely rare entity. We feel that this article may serve as a starting point for further studies to characterize this peculiar entity.
Conflict of Interest DeclarationBoth authors have no conflict or financial interest related to this article.
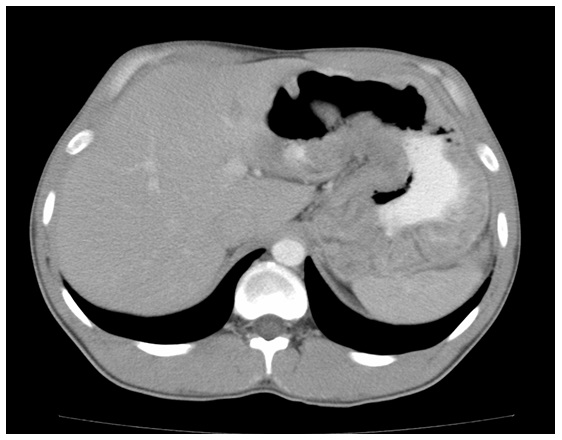
Figure 1: Contrast-enhanced abdominal CT exam demonstrating giant gastric folds involving the entire stomach.
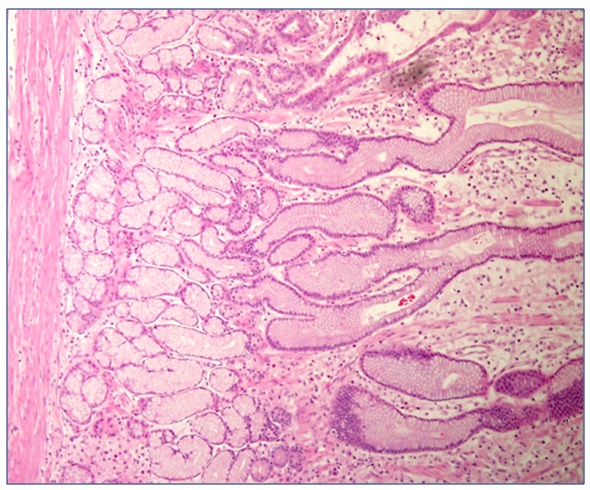
Figure 2: The microscopic findings showed elongated foveolar epithelium with foveolar mucous cell hyperplasia and cystic dilation of the foveolar glands with neutrophil infiltration confined to the muscularis propria. The pathologic diagnosis was Ménétrier disease.
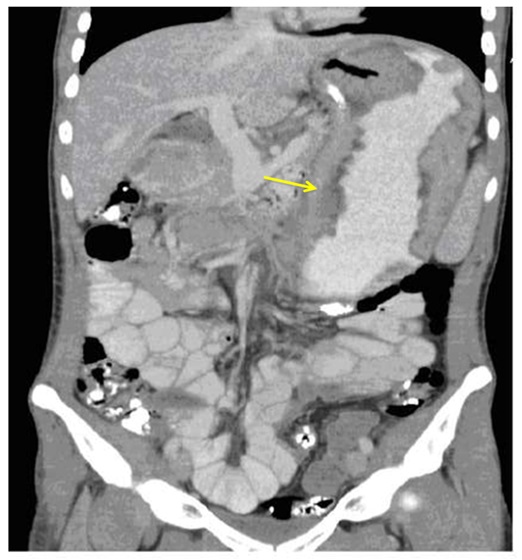
Figure 3: Contrast-enhanced abdominal CT showed marked diffuse thickening of the stomach rugae distributed mainly over the greater and lesser curvatures of the stomach.
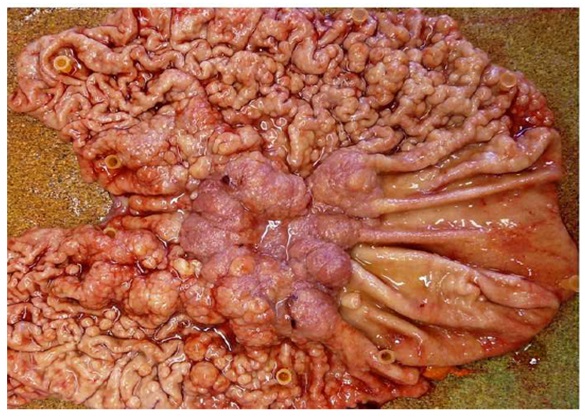
Figure 4: The macroscopic specimen revealed a diffusely edematous and polypoid gastric mucosa with sparing of the greater curvature of the stomach. A focal ulcerated area surrounded by large polypoid lesions, noted at the mid body of the stomach.
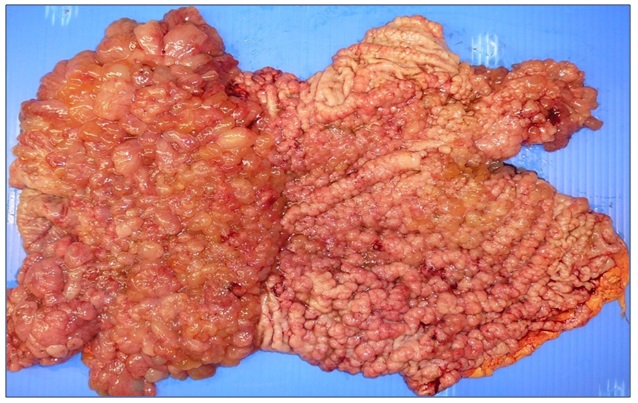
Figure 5: Diffuse polypoid lesions seen throughout the stomach
Chat with us on WhatsApp