
Citation: Abdul L and Sumbul R. Pharmacognostical, Phyto-Chemical and Antibacterial Studies of Some Unani Drugs used in Infectious Diseases. Int J Pharmacogn Chinese Med 2017, 1(2): 000107.
*Corresponding author: Abdul Latif, Department of Ilmul Advia, Aligarh Muslim University, Uttar Pradesh, India; Email: abdullatifamu@gmail.com
Antimicrobial resistance is a serious problem in terms of clinical and public health. In-spite the successes of the Millennium Development Goals since 2000, the inhabitants of low-income countries still suffer of an enormous burden of disease owing to diarrhoea, pneumonia, tuberculosis, malaria, yellow fever, meningitis, HIV/AIDS and other infectious diseases. Regardless of the origin of resistance of antibiotics, the fact is that the number of new resistant microorganisms grows faster than the development of new drugs. Therefore, the search for alternative therapies is imperative. Unani System of Medicine (USM) may be considered as personalized medicine which corrects the imbalance in the four humors- (blood, phlegm, yellow bile and black bile) which occur due to entry of any infective organism in the body. So, drugs correct the imbalance of the humours and aid in regaining health with bactericidal and bacteriostatic activities. Present study provides an in-vitro efficacy of traditionally used medicinal herbs of Unani System of Medicine (USM) viz. Glycyrrhiza glabra Linn. (Asl-us-soos), Cassia fistula Linn. (Maghz-e-Khayar Shamber) and Adiantum capillus-veneris (Parsiyaoshan) in terms of their antibacterial activity against various gram positive and gram negative bacterial strains using Agar well and Kirby Bauer’s disk diffusion method according to CLSI guidelines. The efficacy was compared with the standard drugs used as positive control viz. Ciprofloxacin for gram positive strains and Gentamicin as gram negative strains and the solvent used to dissolve the test drug Dimethyl Sulphoxide (DMSO) as negative control. Pharmacognostical and Phytochemical analysis was also done as review of literature and standardization methods were followed to confirm the authenticity and presence of various phyto-active constituents present in the test drugs. The study demonstrates test drugs to be considered as effective drug target for further screening and its wider use in medicine.
Keywords: Pharmacognostical study; Physico-chemical and Antibacterial analysis, Glycyrrhiza glabra Linn; Cassia fistula Linn; Adiantum capillus- veneris
World Health Organization (WHO) encourages, recommends and promotes traditional remedies in National health care programs as these drugs are easily available at low cost, safe and moreover people have faith in them but also emphasize the need to ensure quality control of medicinal plant products by using modern techniques and applying suitable standards [1]. Herbs are staging a comeback and herbal ‘renaissance’ is happening all over the globe, as herbal products today symbolize safety in contrast to the synthetics that are regarded as unsafe to human and environment.
Long before mankind discovered the existence of microbes, the idea that certain plants had healing potential, indeed, that they contained what we would currently characterize as antimicrobial principles, was well accepted. Plants are appropriate for the treatment of infectious disease for a number of reasons. The evolutionary pressure exerted upon plants by similar pathogens to those commonly causing disease in humans has induced the development of a variety of useful antimicrobial chemicals. These plant antimicrobials offer a great diversity of chemicals for combating infection. Some of them are highly specific in their action in the human body, while others exert a more general influence. Given the alarming incidence of antibiotic resistance there is a constant need for new and effective therapeutic agents. Therefore, there is a need to develop alternative antimicrobial drugs for the treatment of infectious diseases from medicinal plants [2].
Unani System of Medicine (USM) may be considered as personalized medicine which corrects the imbalance in the four humors-(blood, phlegm, yellow bile and black bile) which occur due to entry of any infective organism in the body. So, drugs correct the imbalance of the humors and aid in regaining health. USM has a holistic approach of treatment, utilizes drugs of natural origin used as whole either as powder, decoction or infusion in spite of isolating phyto-constituent of interest which is a common practice in Western medicine. In this provision of USM, the phytochemicals which are believed to play a major role in disease protection appear to work alone and in combination, and perhaps in conjunction, to prevent, halt, or lessen disease. This makes it important to analyze them in drugs of common use. Therefore phytochemical studies are necessary for standardization and also help in understanding the significance of phyto-constituents in terms of their observed activities [3].
USM totally utilized drugs of natural origin used as single (Mufrad) and compound (Murakkab) drugs in health ailments including infectious diseases since long time on the basis of their own long term clinical experience and have mentioned good results in the literature available. But a doubt always remains in mind regarding the scientific revalidation of their therapeutic activities. Hence the present study was carried out to reassess the antimicrobial efficacy of some selected test drugs based on their ethno-botanical literature [4] and use in infectious diseases [5-7] viz. Glycyrrhiza glabra Linn. (Asl-us-soos), Cassia fistula Linn. (Maghz-e-Khayar Shamber) and Adiantum capillus-veneris. (Parsiyaoshan) [8] Focusing to compare their effect upon bacterial growth with the known antibiotics i.e. Ciprofloxacin (for gram positive bacterial strains) and Gentamicin (for gram negative bacterial strains). Furthermore, the study also re-confirms the reported literature claims of therapeutic efficacy and presence of different phyto-constituents having beneficial effect. In Unani Classical literature, they have been reported to be used in infectious diseases like Diphtheria, cold and coryza, sneezing, sore throat and hoarseness of voice etc Figure 1.
Material and MethodsThe drug samples were taken from different sources; Asl-us-soos and Parsiyaoshan were collected from local market of Baradari, Aligarh (U.P) India. Maghz-e-Khayar Shamber was directly collected from plants located at Naqvi Park, Aligarh (U.P) India in the month of MarchApril, 2012. All the drugs were properly identified by the botanical literature available and then confirmed by Prof. S. H. Afaq in Pharmacognosy Section of the Department of Ilmul Advia. Parsiyaoshan was further identified by National Institute of Science Communication and Information Resources (NISCAIR) as Adiantum capillusveneris. (R.No: NISCAIR/RHMD/Consult/2011- 12/1931/23). A herbarium sample was prepared for G. glabra (V.No.SC-0145/14), C. fistula (V.No.SC-0147/14) and A. capillus (V.No.SC-0148/14) submitted in the museum of the Department of Ilmul Advia, Aligarh Muslim University, Aligarh for the future reference.
Pharmacognostical Study of Selected DrugsKnown as Asl-us-soos in Unani and commonly as Liquorice; is the dried unpeeled stolon & root derived from the plant Glycyrrhiza glabra Linn. Liquorice has been used in medicine for more than 4000 years. The earliest record of its use in medicine is found in ‘code Humnubari’ (2100 BC), Assyrian herbal (2000 BC), Theophrastus and Dioscoride [9].
Hippocrates (460 BC) mentioned its use as a remedy of ulcers and quenching of thirst [10]. For many centuries China has used large quantities of liquor rice, and many preparation of it are still used. It plays an important part in Vedic Medicine and is one of the principal drugs of Shusruta. Even today liquorice is maintaining its place in medicine and pharmacy. Its use from then, till today, proves its efficacy.
Botanical description of plant: Indigenous liquorice is obtainable from Baluchistan and North West province of Pakistan. The main supply is imported from the Persian Gulf, Asia Minor, Turkestan, Siberia and also cultivated in China, France, Germany, Italy. It grows to a height of 1 to 2 meter, has dark green spreading pinnate leaves that are divided into pairs of narrow leaflets. The pea-like, purple blue flowers arise from the leaf axils in a spike like cluster. The pods are small and flat, 2-3cm in length, turning brown at maturity and containing 1-7 small dark reniform seeds about the size of a pinhead. The plant has a deep tap root system, produces horizontal stolon and rhizome that spread out from the main plant just under the soil surface, it produces new shoots from buds on the underground stolons [11].
Macroscopy of the root: This drug consist of dried, unpeeled stolon and root having yellowish brown or dark brown outer layer, externally longitudinally wrinkled, with occasional small buds and encircling scale leaves, smoothed transversely, cut surface shows a cambium ring about one third of radius from outer surface and a small central pith; root similar without a pith; fracture, coarsely fibrous in bark splintery in wood; odour, faint and characteristic, taste sweetish [12]. The drug comprises of only root and stolon parts. In the market both peeled and unpeeled drugs are available and used accordingly. Liquorice root usually occurs in the market as 4-5 inches x 2 inches long pieces. The shapes of the peeled and unpeeled pieces are straight and cylindrical. External surface of unpeeled is longitudinally wrinkled covered with a dark reddish brown or purplish brown corky layer, bearing small scars. The outer surface of peeled Liquorice is pale yellow, fibrous and smoothed.
Cassia fistula Linn. (Caesalpiniaceae family)Commonly known as Maghz-e-Khayar Shamber is the pulp of the pod of tree, it is the golden shower; is a moderate size deciduous tree with 12-15 ft high and 3-4 ft in girth, indigenous to India, and naturalized in tropical Africa, South America and West Indies. The drug consists of pulp obtained from fruits which are devoid of seeds, septa and pericarp). It is a safe aperient for children and women even when pregnant, but slows in its action [13]. It is safe purgative drug in Indian and British pharmacopoeias [14]. Medicinally it has been various pharmacological activities like antimicrobial, antifungal, antipyretic, analgesic, larvicidal, anti-inflammatory, antioxidant, antitumour etc.
Botanical description of plant: The tree is native of India and also of Ceylon [15-17]. It is found in Asian, America, Egypt, W. Indies, and Africa [18]. It is commonly found in Malaya, China and Myanmar [13,16]. It is indigenous to India and naturalized in tropical Africa, South America and West Indies. The drug is chiefly obtained from West India and Indonesia. It is also found in Brazil and hot areas of Africa. Arabs and Persians were familiar with this valuable plant and Dymock writes that the drug became known to the latter Greek physicians through the Arabs. It is a tree 8-15 meter high, trunk straight, bark smooth and pale grey when young, rough and dark brown when old, branches spreading, slender. Its leaves 23-40 cm long, leaflets 4-8 pairs, ovate or ovate oblong, bright green and glabrous above, their leaflets are in 4-8 pairs, petioles 6-10 mm long, pubescent or glabrous. Its flowers are in racemes 30-50 cm long, pedicels 3.8-5.7 cm long, slender, pubescent or glabrous. Pods 30-60 cm long, 2-2.5 cm diameter, pendulous, cylindrical, nearly straight, smooth, shining brown black indehiscent with numerous (40-100) horizontal seeds immersed in a dark coloured sweetish pulp, and completely separated by transverse dissepiments. Seeds are broadly ovate 8 mm long slightly less in breadth, and 5 mm thick [16,19].
Macroscopic characteristics: Fruit contains many celled indehiscent pod, 35-60 cm long and 18-25mm in diameter, nearly straight and cylindrical, chocolate brown to almost black in colour, pod surface smooth to naked eye, but under lens showing minute transverse fissures; both dorsal and ventral sutures, evident but not prominent, short stalk attached to base of fruit and rounded distal end mucronate, pericarp thin, hard and woody; fruit initially divided by transverse septa about 5mm, apart each containing a single seed attached to vertical suture by a long dark, thread like funicle about 8- 12 by 6-8 mm, circular to oval, flattened reddish-brown, smooth, extremely hard and with a distinct dark brown line extending from micropyleto base, seed initially embedded in a black viscid pulp consisting of black, thin, shining, circular disc like masses having central depression of seed on both surface or as broken piece adhered with each other.
Adiantum capillus veneris (Polypodiaceae family)Known commonly as Parsiyaoshan in USM; Adiantum constitutes of about 200 species of ferns under this genus, occurring in tropical America. Some species are hardy, while others require protection. The Greek word adiantos, means dry, the genus so named because leaflets shed water in a remarkable way, if plunged into water the fronds remain dry. Leaves are thin, simple or divided into fan shaped pinnules. Its petioles are black or purplish and shiny. It need compost mixture consisting of one part each of loamy soil, silver sand and charcoal and two parts leaf mould is suitable for their growth. It is propagated by spores which is shown on fine sandy leaf mould and kept moist and covered by glass. These are also multiplied by division of clumps [20].
Various species of Adiantum are recorded as used in Traditional Medicine in Europe, Saudi Arabia, Africa and the Indian Subcontinent. They contain hopanetriterpenoids, a group of squalane – derived compounds more commonly associated with bacterial membranes.
Botanical description of plant: A delicate fern of damp places found chiefly in the western Himalayas, ascending up to an altitude of 2,400 meters and extending to Manipur. It is common in Punjab, Bihar, Maharashtra and South India [21]. It is also found in Madras Presidency, west side up to 5,000 feet as the mountains Ceylon, N. India, Europe, Africa, America and Australia [22].It is also found in Iran, Afghanistan and Pakistan. It is known as Maiden hair fern. It is chiefly obtained in the Punjab Market and can also be had in some of Southern India. The expressed juice with pepper is a favorite remedy in all kinds of fever. Syrup prepared from the leaves is useful in chronic cough [10].
Macroscopic characteristics: Stipes are suberect, rather slender 10 to 23 cm long, polished, blackish, naked, fronds bipinnate, with a short terminal pinna and numerous erect patent lateral ones on each side, the lowest slightly branched again, segments 1.3 to 2.5 cm broad, the base cuneate, the outer edge rounded, deeply lobed from the circumference in the direction of the centre and the lobes again bluntly crenated, lowest petioles are 6mm with pellucid texture, herbaceous with thin rachis and both surfaces naked, placed in the roundish sinuses of the crenations [16].
Phytochemical StudiesGlycyrrhiza glabra Linn. (Asl-us-soos): The chief constituent of Liquorice is “Glycyrrhizin” which is present in the drug in the form of potassium and calcium salts of glycyrrhizic acid. It is a yellow amorphous powder sweet in taste and soluble in water. Glycyrrhizic acid is not a glycoside since it yields on hydrolysis one molecule of glycyrrhetic acid and two molecules of glycuronic acid but no sugar. It also contains Glucose 5 to 15 % , Sucrose 2.4 to 6.5 % & Fat 0.8 %, Asparagine 1 to 2%, Glycyramarin, β sitosterol, Protein, Starch (29%), Flavanoids like Liquirtin , Isoliquirtin , Liquiritigenin , and Isoliquiritigenin, Volatile compound 0.04 to 0.06 % acid, resin, gum, mucilage, phosphoric acid, sulphuric acid, mallic acid, cadmium and magnesium salt [23] Isoflavones, glabridin, hispaglabridins A & B and glabrene [24]. Coumarins present in G. Glabra include liqcoumarin, glabrocoumarone A & B, Herniarin, Umbelliferone, Glycyrin, Glycocoumarin, Licofuranocoumarin, Licopyranocoumarin. The presence of many volatile components such as pentanol, hexanol, linalol oxide A and B, tetramethylpyrazine, terpinen-4-ol, α-terpineol, geraneol and others in the roots are reported. Presence of Propionic acid, benzoic acid, ethyl linoleate, methyl ethyl ketine, 2, 3-butanediol, furfuraldehyde, furfurylformate, 1-methyl-2-formylpyrrole, trimethylpyrazie, maltol and any other compounds are also isolated from the essential oil [9].
Cassia fistula Linn. (Maghz Khyaar Shambar): By steam distilling the finely powdered fruit, a dark yellow volatile oil with honey odour is obtained. Water which distills over with the oil contains normal butyric acid [20,25]. Tannic acid, aluminous matter, starch, oxalate of calcium, proteins (19.94%) and carbohydrates (26.30%), arginine, leucine, methionine, phenylalanine, tryptophan, aspartic and glutamic acids, a new dimericproanthocyanidin CFI isolated along with (-) epiafzelechin, (+) catechin, kaempferol, dihydrokaempferol and 1,8-dihydroxy-3- methylanthraquinone and its structure was determined [26]. Phenolics, proteins, steroids, aluminium, calcium, iron magnesium, sodium, potassium [25]. Volatile oil, waxy and resinous derivatives were isolated from the fruit of the plant [25,14]. A major anthraquinone derivative called rhein is isolated from the fruit pulp of the plant [26,27]. A compound called 1, 8-dihydroxy-3- anthraquinone derivative in fruit pulp of the plant was isolated [27]. Glucose, sucrose & fructose are also isolated from fruit pulp [23].
Adiantum capillus veneris (Parsiyaoshan): It consists of a bitter principle capillerin, volatile oil, Tannin, Mucin, Terpenoids, Filicinuic acid, Gallic acid, sugar. A mixture of esters, Ketone (m.p. 222oC), a diol (m.p. 243°C), a nortriterpeneadiantonetriterperne epoxide- adiantoxide (m.p. 229oC) isolated & characterized as 3α, 4α,- epoxyfilicane. Astragalin, isoquercitrin, Nicotiflorin, Kaempferol 3-o-glucuronide, Rutin and quercituroneare are isolated [28]. Kaempferol-3-o-rutinoside sulphate, 1- caffeylglucose and sulphate esters of 1 coumaryl galactose isolated from fronds; kaempferol-3-sulphate isolated and characterized.Quercitin-3-o-(6’’-malonyl)-D-galactoside (i) isolated and its structure elucidated. Diacylglyceryl-o- 4’ (N,N,N-trimethyl)-homoserine isolated from fronds [26]. Isolation of beta sitosterol, stigmasterol and campesterol from leaves [28].
Physico-chemical studies: Phytochemical studies of the plant preparations are necessary for standardization, which helps in understanding the significance of phytoconstituents in terms of their observed activities. Phytochemistry also helps in standardizing the herbal preparations so as to get the optimal concentrations of known active constituents, and in preserving their activities [29]. Physico-chemical analysis in terms of their organoleptic features, extractive values, bulk density, moisture content, solubility, ash value, pH analysis at 1% and 10% was done as per the Pharmacopoeial guidelines Qualitative analysis of the chemical constituents present in the drug samples was also done as per the guidelines [30] (Table 1-5).
Anti-Microbial activity: The present study was conducted in the Microbiology Laboratory, Department of Ilmul Advia, Faculty of Unani Medicine, AMU, Aligarh in the month of August-September, 2012; with an objective of screening the antimicrobial activity of three drug viz. Asl-us-soos, Maghz-e-Khayar Shamber and Parsiyaoshan, which includes its antibacterial activity in terms of Zone of Inhibition (ZOI in mm) of their hydro-alcoholic extracts of the selected test drugs for the clinical isolates of the bacterial strains obtained from Department of Microbiology, Jawaharlal Nehru Medical College AMU, Aligarh.
Preparation of the test drug extracts: Extraction was done according to the method described by [30] with some minor modifications, keeping in mind that the thermo labile elements present in the drugs are destroyed when exposed to a higher temperature beyond 55°C, so the heat wherever was needed was kept as low as possible to prevent the loss of thermolabile substances, present in the drugs from destruction. Strict aseptic precautions were followed throughout the process. For hydroalcohalic extract coarse powdered drugs were extracted using soxhlet apparatus, by reflux method with hydroalcohalic 95% Ethanol and Distilled water in a ratio of 1:1 as a solvent at 50°C for 6 hours or until the extracting return in the siphon was colourless. The extract obtained was subjected to dryness in the Lypholizer (Macro Scientific works, New Delhi) under reduced pressure. The respective stock solutions so prepared were refrigerated till further use. It was redissolved in DMSO at the concentration of 5mg/ml and 50 µl is from each stock solution were used.
Antibacterial susceptibility testing: Antimicrobial susceptibility testing was done by Kirby Bauer’s disk diffusion method [31] as was performed on biofilm isolates by Kirby-Bauer on Muller Hinton Agar [32]. The standard medium Mueller Hinton Agar, was poured to a depth of 4 mm in a 90 mm petridish. The plate was inoculated by streaking the entire surface in three planes with a sterile cotton swab (PW041, Himedia Labs Pvt. Ltd., Mumbai, India) dipped into standardized inoculums, spreaded evenly with the help of L-Spreader (PW1085, Himedia Labs Pvt. Ltd., Mumbai, India). The bacterial inoculum was prepared from an 18 hour broth culture of the microbe to be tested and was standardized with sterile physiologic saline to contain 106cfu/ml. Standardized commercial paper disk containing amounts of the antimicrobial agents to be tested were placed on the surface of the agar. The plate was incubated in an inverted position at 37°C for 18 hours. The diameter of zone of inhibition produced by the test drugs, positive control (ciprofloxacin for gram positive bacterial strains and gentamicin for gram negative bacterial strains) and negative Control (DMSO) was measured with the help of Antibiotic zone scale (PW297, Himedia Labs Pvt. Ltd., Mumbai, India) from each disk [33].
Statistical AnalysisAll the statistical analysis was done using G-paid software, One way ANOVA and the post-test named Bonferroni: Selected pairs of column with multiple comparison was performed with p-value <0.05.
Observation and ResultsPhytochemistry also helps in standardizing the herbal preparations so as to get the optimal concentrations of known active constituents, and in preserving their activities [29]. Physico-chemical analysis in terms of their organoleptic features, extractive values, bulk density, moisture content, solubility, ash value, pH analysis at 1% and 10% was done as per the Pharmacopoeial guidelines and the results are depicted in Tables 1-5. Qualitative analysis of the chemical constituents present in the drug samples [30] was done. Standard tests and methodology has been followed to confirm the presence of chemical constituents (Table 6) while the zone of inhibition determined as a part of antimicrobial analysis in Table 7.
DiscussionThe term antibacterial is consequently used to include those active compounds prepared synthetically or isolated from higher plants. Unfortunately, due to the widespread and often indiscriminate use of antibiotic together with poor hygiene, many pathogenic organisms have acquired a resistance to specific antibiotic treatment and these strains are particularly evident in the hospital environment. The problem is further compounded by the fact that resistance to a particular antibiotic can be transferred from one organism to another. Thus the clinician’s antibiotic armamentarium is being steadily eroded and research directed towards the isolation or synthesis of new drugs is still a matter of considerable urgency. During the last two decades there has been a tremendous progress in development of synthetic as well as semi synthetic drugs which possess antibacterial activity. Although much attention has been paid to develop the potent antibacterial drugs with fewer side effects and adverse reactions, relatively a few of them are really free from adverse effects. Several medicinal plants have been observed to possess antimicrobial activity. This review has highlighted some of the medicinal plants as means to contribute towards the development of effective and safe antimicrobial agents.
Present study demonstrated the ethno-botanical literature along with the phyto-chemistry of the selected test drugs while physic-chemical analysis and antimicrobial study has been done as a means to contribute towards the development of effective and safe antimicrobial drugs in future.
ConclusionAn antibiotic assay done in the study reveals that selected test drugs viz. G. glabra, C. fistula and A. capillus have a significant antibitotic activity, most significant sensitivity was shown towards S.aureus; individually it was observed that all the strains used are sensitive to G. glabra and K. pneuomoniae was resistant towards it; S .aureus, S. pyrogenes, C. diphtherium, S. viridans, P. vulgaris were sensitive to C. fistula while for A. capillus only S. aurues and S. mutans were sensitive while among the gram negative strains it showed a resistant pattern to all bacterial strains except E. coli. However this data is just a step ahead regarding their scientific validation. Many other studies are needed in this direction to ensure its use clinically to combat infectious diseases caused by such bacterial species.
Unani medicine claims to possess a large number of such effective and safe drugs useful in the treatment of infectious diseases that are successfully in use since centuries for the treatment of infectious diseases with a good recovery rate and have not been found or reported to cause any major side effects. Researchers in this field should give this priority to the study of those drugs which are lacking in western countries. Our ancestors of Unani medicine did not only discover active plants/ mineral, but they also developed a holistic approach in their system.



Figure 1: Selected traditional medicinal drugs used in Infectious diseases.
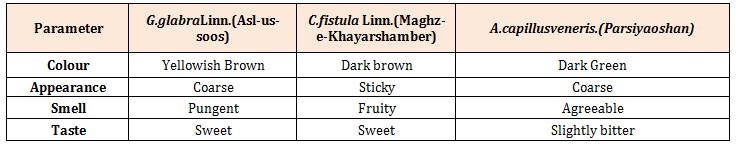
Table 1: Organoleptic Characters of powdered Drug.
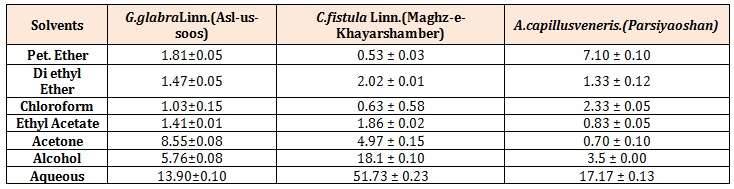
Table 2: Percentage of Extractive Value in different Solvents (Mean ± S.E.M).
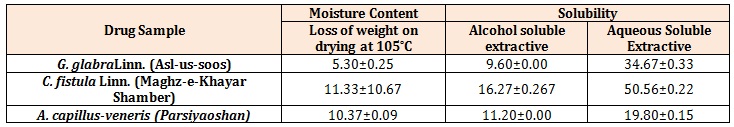
Table 3: Percentage of Moisture Content and Solubility.
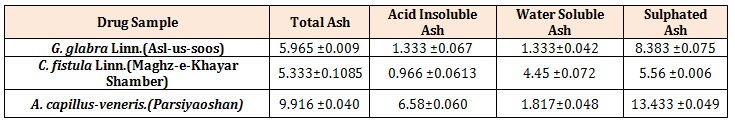
Table 4: Percentage of Ash Value.
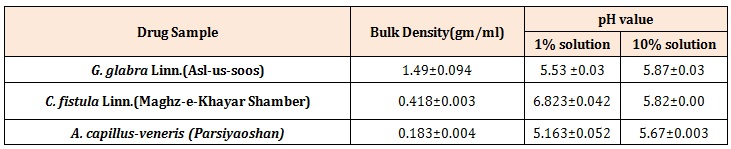
Table 5: Bulk Density and pH value.
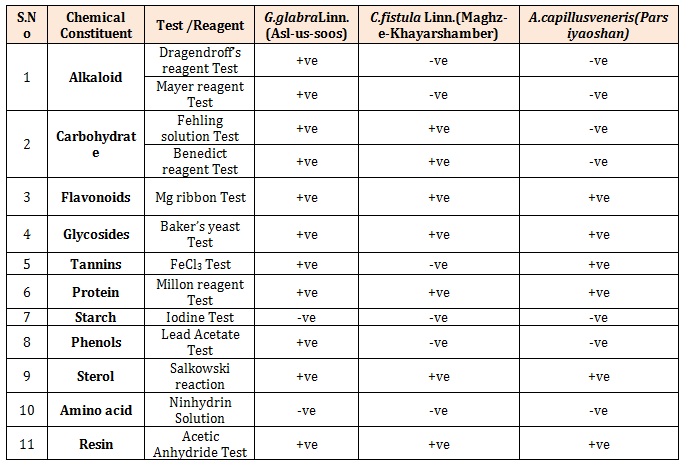
Table 6: Qualitative Analysis of the Phytochemicals(-ve=Not present; +ve=Present).
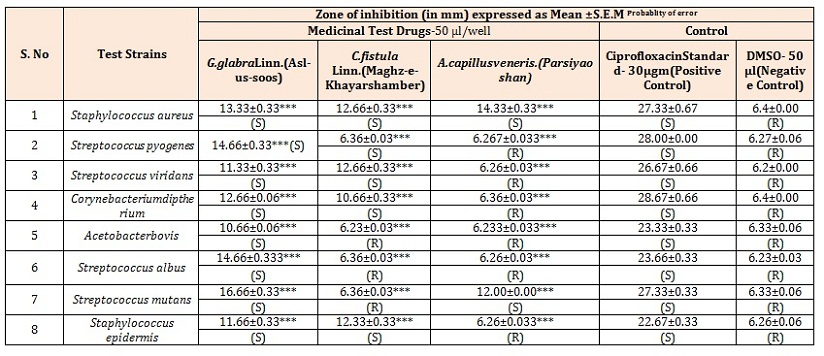
Table 7: (a) Antibacterial Activity of test drugs against Gram positive Bacterial Strains.
S= Sensitive to bacterial Strain; R=Resistant to bacterial strain; Significance: *** = p < 0.001, ** = p < 0.01; * = p < 0.05.
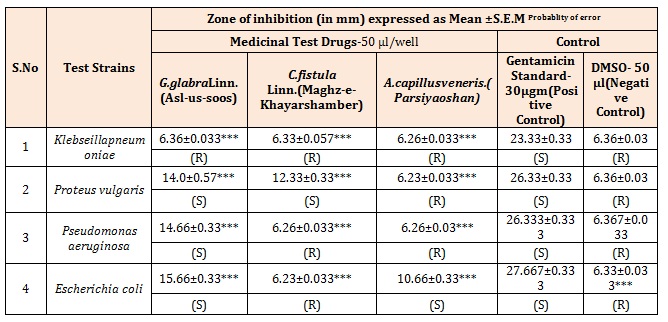
Table 7: (b) Antibacterial Activity of test drugs against Gram negative Bacterial Strains.
S= Sensitive to bacterial Strain; R=Resistant to bacterial strain; Significance: *** = p < 0.001, ** = p < 0.01; * = p < 0.05.
Chat with us on WhatsApp