
Citation: Harry S. Tranexamic Acid in Total Knee Replacement Reduces Wound Complication and Transfusion Rates; An Observational Study. J Ortho Bone Disord 2017, 1(5): 000129.
*Corresponding author: Harry Sargeant, NHS Grampian, Aberdeen Royal Infirmary, Aberdeen, AB252ZN, UK, Tel: 00447583011277; Email: harrysargeant@Gmail.com
Tranexamic Acid (TXA) has been shown to reduce transfusion rates in Total Knee Replacement (TKR) without complication. We undertook a cohort study investigating TXA use with TKR, wound complication and transfusion rate. We added intravenous TXA to our standardised TKR protocol for 6 months of a 12-month study period, with no other changes during this time.
MethodsAll patients undergoing primary TKR over the 12-month study period were identified. Notes and online records were reviewed to collate details including demographics, wound complication, blood transfusion, length of stay, and haemoglobin levels. All study patients received a Columbus navigated TKR, and routine thromboembolic and antibiotic prophylaxis.
Results124 patients were included, 72 receiving TXA. A significant change in wound complication was noted; 15% of patients (n=11) of the TXA group had a wound complication, with 40% of patients (n=21) in the control group (p = 0.003). All four patients requiring a blood transfusion were in the control group (p = 0.029). There was no diagnosed pulmonary embolusor mortality.
ConclusionIn our unit we have demonstrated a significantly lower transfusion rate, wound complication rate and length of stay, without any significant increase in thromboembolic disease with the use of TXA in TKR.
Keywords: Tranexamic Acid; Total Knee Replacement; Surgical Wound Infection; Blood Transfusion
Tranexamic acid (TXA) has a broad spectrum of use and has been demonstrated to significantly reduce blood transfusion in cardiac, cranial, orthognathic, hepatic, urological and orthopaedic surgery [1]. Its use within orthopaedic surgery as an anti fibrinolytic is well demonstrated. Meta-analyses in both Total Hip Replacement and Total Knee Replacement (TKR) have shown significantly fewer blood transfusions with tranexamic acid (TXA) with no significant increase in post-operative complications, including venous thomboembolism [2-4].
The benefits of reducing blood transfusion rates include lower cost, shorter length of stay, improved mobility and avoiding the risks of transfusion reaction and transfusion transmitted infection. There is also an increased risk of deep prosthetic joint infection with blood transfusion [5].
Chemical thromboprophylaxis in the form of low molecular weight Heparins, Aspirin and newer agents such as Rivaroxaban is widespread in TKR and most joint replacement surgery. It is recommended for a minimum of 10 days post operatively by the American Society of Chest Physicians [6]. National Institute for Clinical Excellence (NICE)guidelines also recommend low molecular weight heparin (LMWH) for all in patients undergoing orthopaedic surgery unless contra-indicated [7]. This thromboprophylaxis carries with it the increased risk of post-operative bleeding. There is a relative paucity in the literature on the impact of thromboprophylaxis on wound healing compared with its impact on reducing venous thromboembolism. Questionnaires however, have shown that a large proportion of surgeons recognise this, and have discontinued thromboprophylactic regimes in the case of local wound complications such as haematoma, bruising and delayed healing [8]. It is logical that local wound issues have an impact on infection rates and it has been demonstrated that wound complications are increased in patients receiving thromboprophylaxis including LMWH and Warfarin [9,10].
As TXA reduces bleeding and therefore blood transfusion rates, it seems plausible that it should improve the appearance of wounds post-operatively.TXA has been shown in breast cancer surgery to reduce local wound complication rates [11]. This effect on wound complication has not been investigated in orthopaedic surgery. Some studies have shown superficial wound infection rates in TKR to be as high as 10.5% which in turn is a risk factor for deep prosthetic infection, a disastrous complication following joint replacement surgery [9]. It was with this in mind that we propose that wound complications may be reduced with the use of TXA through its antifibrinolytic action, thereby reducing postoperative bleeding and localized haematoma. In the same way anticoagulation can lead to wound complications, an antifibrinolytic could have a positive impact on wound appearance and a subsequent impact on infection rates. Our aim was to investigate whether TXA has an effect on wound complication and transfusion rates.
Over a 12 month study period we added one gram of TXA to the standard peri-operative management of TKR patients. We subsequently analysed the impact of this with regard to wound complication rates, haemoglobin levels and transfusion rates. There were no other changes to the routine management of patients during the study period.
Our null hypothesis was that TXA would have no effect on wound complication and transfusion rate.
AimTo determine the effects of Tranexamic Acid on wound complication and blood transfusion rate after Total Knee Replacement.
MethodsAll patients undergoing primary TKR from June 2014 until 2015 in our unit were identified. Case notes were reviewed and a standardised proforma was used to collate the following information; patient demographics, date of surgery and admission, operative details, thrombophrophylaxis, prophylactic antibiotics and tranexamic acid use. Primary outcomes investigated were documented wound complication, transfusion rate, and mortality. Secondary outcomes included venous thromboembolism, myocardial infarction, cerebrovascular accident, additional investigations including post-operative duplex ultrasound, CT Pulmonary Angiogram, pre and post-operative Haemoglobin. The Picture Archiving and Communication System (PACS) were used to review any imaging and the results of this. Online electronic records were then also reviewed to corroborate paper notes and review haemoglobin levels, further admission episodes and investigations. Any review within six weeks postoperatively was considered early and a ‘complication’. All other complications within the first six weeks were documented. All patients were enrolled in a standardised enhanced recovery programme; including same day mobilisation if possible, analgesia commenced the night before surgery, spinal anaesthetic, local infiltration of Ropivacaine 0.2% and a standardised post-operative analgesic regimen. TXA, was added to this programme 6 months into the study and was given intravenously during surgery at a dose of one gram.A wound complication was defined as any wound issue that caused a deviation from the usual post-operative course. This had to be documented by a medical practitioner and could include an additional ultrasound scan, blood investigations, unplanned early review or simply observation. If a patient had an unplanned review in the community for their wound this was also documented as a wound complication.
We only included patients who received the unit’s standard primary TKR (Columbus Navigated TKR through a medial parapatellar approach with a tourniquet), 14 days low molecular weight heparin (Dalteparin) commenced 6 hours following surgery and intravenous antibiotic prophylaxis. Exclusion criteria were use of a drain, alternative thromboprophylaxis, any coagulation disorder and previous venous thromboembolism.
Summary statistics including mean and standard deviation were calculated. Statistical analysis was performed on the data, with unpaired two-tailed Students T test for continuous variables, and Fishers exact test for categorical variables. A P value of less than 0.05 was considered significant. Relative risk (RR) ratio was calculated using inverse variance method with a fixed effects model with 95% confidence interval (CI). All statistical analysis was performed using IBM SPSS software version 20.0 (IBM-SPSS, Chicago, IL, USA) Subgroup analysis was performed for wound closure technique, gender and lead surgeon.
ResultsA total of 130 patients underwent TKR between June 2014 and June 2015. Six were then excluded due to Warfarin thromboprophylaxis as per exclusion criteria. Those remaining received Dalteparin for 14 days post operatively. All patients underwent a primary procedure with Columbus® navigated prosthesis (B. Braun Aesculap, Tuttlingen, Germany). All patients received intravenous antibiotics prior to insufflations of the tourniquet and for 24 hours following the procedure.
A total of 124 patients were included, with 72 receiving TXA and 52 not (control group). Mean age was 70(48-88) with 67 males. There was no significant difference between the age (p = 0.22) or pre-operative Haemoglobin status of the TXA and control group (p = 0.65). (Table 1)
Primary Outcome Measures15% of patients (n=11) of the Tranexamic acid group had wound complications, with 40% of patients (n=21) in the control group (p = 0.003) risk ratio (RR) 0.38, 95%CI (0.2 to 0.71); risk difference -0.25, CI -0.41 to -0.09. The number needed to treat was four. Wound complication breakdown is illustrated in Figure 1. Four patients required a blood transfusion, all of whom were in the control group (p = 0.029). There was no mortality.
Secondary Outcome MeasuresMean change in Haemoglobin was 22 in the control group and 21 with TXA (p = 0.859). Mean length of stay was 6.83 days in the control group and 5.15 with TXA (p < 0.001). There was one patient with a deep vein thrombosis confirmed by ultrasonography in the TXA group. One patient was taken back to theatre for a washout of their wound from the control group, and subsequently diagnosed with deep infection. Further management of post-operative complication is demonstrated in Table 2. No patients were documented as having a suspected or radio logically confirmed pulmonary embolus. There was no other significant postoperative complication in either group including myocardial infarction or cerebrovascular accident. There was no significant difference in wound complication and transfusion rates with wound closure method (subcuticular suture vs staples) or lead surgeon (p=1.00).
DiscussionThe first of our primary objectives with this study was to determine if TXA had any effect on wound complication. There has been well documented evidence of the effect on transfusion rates of TXA as mentioned [1,2]. There has, however not been any published study on the effect of wound healing and appearance with the use of TXA. High post-operative INR and aggressive anticoagulation have been associated with increased infection rates, and this logically seems to be due to the local inhibition of clotting, subsequent wound haematoma, ooze, and swelling. These findings in the wound are themselves associated with development of superficial surgical site infection [12,13]. The presence of superficial surgical site infection has been associated with the development of deep prosthetic infection [14,15]. One study demonstrated double the infection rate in patients treated with warfarin compared with no thromboprophylaxis [16].
Deep prosthetic infection is a devastating complication of TKR. With the recommendation to use thromboprophylaxis unless contra-indicated in all joint replacement surgery it could be that use of an antifibrinolytic can help improve wound healing post operatively, and perhaps counteract some of the downsides of aggressive anticoagulation [6,7]. The effect of TXA on wound complication and healing has been investigated in other types of surgery where it has been shown to be beneficial [11]. It was with this in mind that we sought to look at its effects on wound healing in TKR. These results have demonstrated that TXA has had a positive effect on wounds post operatively, with a significant reduction in documented complication; particularly excess swelling and suspected superficial infection. Larger longer term studies would be needed to examine whether this benefit has an impact on deep infection rates and prosthesis survivorship.
The other primary objective of this study was to confirm whether TXA has some effect on transfusion rate. We have observed a significant reduction in transfusion rate without a detected increase in complication. This effect of TXA on reducing surgical bleeding and transfusion rates has been well demonstrated throughout the literature in TKR and also across numerous surgical specialties [1,2]. We can further add to this established hypothesis showing that it is efficacious in our centre. The benefits of reduced allogenic red blood cell transfusion rates are far reaching. Reduced cost for the hospital in use of expensive and precious donated blood, shorter inpatient stay and avoiding delay to physiotherapy. Further patient benefits include avoidance of exposure to possible viral and bacterial infection, transfusion reaction and rare possibilities such as prion disease and variant Creutzfeldt-Jakob disease [17].
There has been some concern that blood transfusion itself is an independent risk factor for post-operative surgical site infection. Studies have demonstrated this in primary arthroplasty, spinal surgery and general surgery [18-20]. This may be due to contaminated products, however with modern testing methods this should be uncommon. It has also been shown that allogenic transfusions have a higher associated risk of infection than of autologous transfusion [5]. It may therefore be an immunomodulatory effect of the transfusion rather than contamination which is causal. It has been suggested that this is due to impairment of Natural Killer T cell function and antigen presentation, or may be due to graft versus host disease [21,22].
Further to this we would argue that the improved appearance of the wound and lower wound complication rates in the short term is a demonstration of the local antifibrinolytic effect of TXA. Those patients who have prolonged bleeding and wound ooze would have an increased risk of local, superficial and therefore subsequently deep infection. These patients who are losing blood through their wound or into the knee joint are both those who are more likely to require a transfusion, and those who will have problematic wounds post operatively and therefore be at increased risk of infection. We would argue that TXA is beneficial in local wound appearance and the manifestation of excessive surgical bleeding leading to transfusion, both independent risk factors for infection.
We found no evidence of increased rates of thromboembolism, either arterial or venous. With the size of our study we cannot scrupulously demonstrate the safety profile of TXA, but simply have not shown any concerning increase in Cerebrovascular Accident or Myocardial Infarction post operatively. We acknowledge the limitations in size and retrospectivity with this study and therefore draw conclusions that should be clarified with further prospective randomised study. This may be difficult to justify, however, given the existing widespread evidence for the use of TXA in joint replacement surgery. Further limitations acknowledged within this observational study include; no standardised transfusion threshold, no randomisation of participants, and no blinding of operative surgeon to TXA use. Subgroup analysis however revealed no significant differences between lead surgeon and method of closure. Those diagnosing wound complications, although not blinded, were not included within the research group and completely unaware of the study.
The significantly reduced rate of antibiotic prescribing is a further benefit of TXA that we have demonstrated. Wounds following TKR can often be warm and erythematous as part of the normal post-operative appearance due to local tissue irritation and damage from surgery with subsequent inflammation and healing. We felt there was an over-treatment of suspected superficial SSI due to the inflamed appearance of the surgical site, and this was leading to an elevated rate of antibiotic prescribing in the community, often by non-orthopaedic practitioners not familiar with these wounds. Despite some of these cases probably not being genuine superficial surgical site infection, there is still significant morbidity when they are treated as such. They are a burden on the healthcare system, cause undue anxiety and stress in patients, and result in unnecessary antibiotic treatment. Antibiotic over-use has contributed to multiresistant organisms, and can cause undue side effects in patients [23,24].
Our rates of wound complication were very high, particularly in the control group. This is probably due to the way we classified this, including those diagnosed by community medical practitioners. These wound complications were most probably benign, representing more prominent inflammation than usual, and did not require intervention. Although these cases may well have settled with time, these are still concerning for both patient and practitioner. This is reflected in the higher antibiotic prescribing rates which were probably for inflamed wounds rather than treatment of true surgical site infection. The findings of this work have been shared locally to address this, and all wound concerns are now seen directly by the Orthopaedic department.
ConclusionBased on the results of this observational study, we can conclude that the use of TXA significantly decreased the rate of wound complication and blood transfusion within our unit. The results of this study highlight the requirement for further double-blinded randomised controlled trials investigating the use of TXA in the prevention of post-operative wound complication in primary TKR.
DeclarationThe Authors declare that there is no conflict of interest. Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This manuscript has been seen and approved by all authors who are fully conversant with its contents. All data was collected using the NHS Grampian Clinical Effectiveness Tool in accordance with local policy.
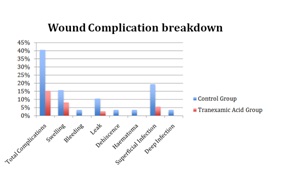
Figure 1: Breakdown summary of wound complications.
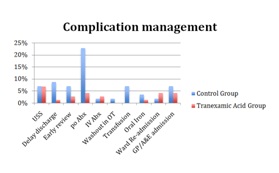
Figure 2: Breakdown summary of wound complication management.
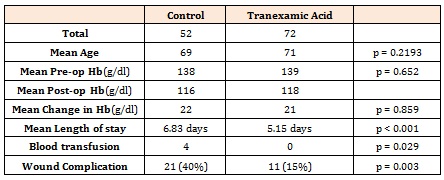
Table 1: Demonstration of post-operative complication.
Chat with us on WhatsApp