
Citation: Ameer Hassan TA. Development of Nanosensors in Nuclear Waste Water. Nanomed Nanotechnol 2016, 1(1): 000104.
*Corresponding author: Thamir Abdul Ameer Hassan, Department of Physics, Chancellor of Al-Karkh University of science, Iraq ; Email: President@kus.edu.iq; Thamir_h@yahoo.com
Studying the bonding nature of uranyl and Plutonyl ion and graphene oxide (GO) is very important for understanding the mechanism of the removal of uranium from radioactive waste water with GO-based materials, lead to the principle of nuclear nanosensor. This article highlights the work of nanosensors in dealing with nuclear waste water discovered by interaction of Graphene and Graphene oxide with uranium and plutonium and their complex.
Keywords: Nuclear energy; Graphene oxide (GO); Radioactive Materials; Uranium; Plutonium
As the theme of this article, I have chosen the words by Richard Feynman: “I will not now discuss how we are going to do it, but only what is possible in principle - in other words, what is possible according to the laws of physics” [1]. Just after Christmas 1959, he delivered a now-famous talk – titled “There's Plenty of Room at the Bottom” - at the California Institute of Technology. It is possible, he proposed, for scientists to assemble new materials at the level of single atoms and molecules, where there are “new kinds of forces and new kinds of possibilities, new kinds of effects”. It is generally accepted that Feynman's visionary discussion of the problems and promise of miniaturization constituted the starting point for the new field that today is called Nanotechnology.
With the rapid development of Nuclear energy, radioactive wastewater has become of major concern and environmental challenge throughout the world; Uranium and Plutonium are the naturally occurred elements in nuclear energy programs. In the meanwhile, large amounts of Uranium have been inevitably released into the environment which would cause serious health problems due to its extremely chemical and radioactive toxicity [2]. Uranium and Plutonium ions and their complexes play an important role in nuclear fuel reprocessing, and their trace characterization is important in nuclear forensics. Uranium and Plutonium oxides and their complexes are major Constituents present in soils and groundwater around contaminated sites, as well as, near production facilities [2-4].
Dissolved Uranium is predominantly present in the form of Uranyl (VI) complexes. 1 Plutonium is known to exist in aqueous solution in four different oxidation states, III, IV, V, and VI, with the last two as the dioxo-species PuO2+and PuO22+ [5]. The Uranyl ion in aqueous phase is reported to form pentagonal bipyramidal structure with five water molecules lying in equatorial plane. In the presence of counterions (CO3 2−, OH−, and NO3−), one or two water molecules in the first shell are replaced by the counterions preserving five-fold symmetry. Unlike uranyl ions, U3+, Pu3+, and Pu4+ form coordination with water molecules [6,7]. There is a strong electron transfer observed, and counterions are strongly bonded to the central actinide ion. The contamination of groundwater due to accidental leakage of radioactive wastes poses a grave danger to the environment and human life. Hence, trace characterization of these radioactive materials is essential in nuclear forensics. The design and fabrication of electronic molecular devices as sensors for Plutonium and Uranium using theoretical and experimental studies has gained paramount importance in the nuclear community.
Recently, it has been found that flakes of graphene oxide (GO) added to water caused radio nuclides to condense into clumps [8]. Experiments were conducted with water containing several actinides including U and Pu along with Ca and Na to study their interaction with GO. GO is shown to be more effective in removal of radionuclides compared with traditional bentonite clays and activated carbon. 11 GO can be prepared by oxidizing a large flake of graphite using Potassium permanganate in the presence of sulfuric and phosphoric acids [9]. GO is partially oxidized and highly amorphous; due to random arrangement of functional groups and large structural disorder in the carbon skeleton, detailed atomic structure is still elusive. Common functional groups reported on oxidation of graphene are −COOH, −OH, −C=O at edges, and C−O−C and –OH on the basal plane [8-11].
When these Uranyl or Plutonyl complexes interact with a graphene molecule (Figure 2), the Pu−O and U−O bond lengths increase, thus indicating weakening of these bonds due to interactions of the ctinide ions with graphene. (Table1) lists the bond distances of some U/Pu complexes for comparison purposes.
Now discussion molecular electrostatic potential and interaction of Graphene Oxide with U and Pu Complexes
Molecular Electrostatic Potential (MEP)Solid surface colors are indicated to potentials as
(i) Blue is the most positive potentialMEPs of U and Pu complexes adsorbed on graphene illustrated in (Figure 3). Graphene electrical and optical responses depend on the interaction of those plasmons with the moieties attached to the graphene surface; we suggest that plasmons are highly sensitive and are likely to be affected by the electrostatic field of a single molecule. A potential challenge for the detection is that some waters are closer to graphene than are Pu or U, indicating the need for graphene functionalization to enhance sensitivity.
Graphene Oxide Interaction with U and Pu ComplexesThe interaction of plutonium complex with GO has been studied using two types of structure of GO by Narendra Kumar and Jorge M. Seminario [12] (Figure 4a,b). As indicated above, when graphene is oxidized to form GO, random arrangement of different types of functional groups on the graphene sheet due to partial oxidation was found. The researchers were used:
(i) A GO with two −OH and one −O− functional groups present or type I andThe mechanism of sensing noted that introduction of −CO group on GO leads to breaking of few bonds, and hence those C atoms are saturated with H atoms to satisfy the valence of carbon atoms.
The plutonium complex used plutonium nitrate with few water molecules surrounding it. Nitrates form very strong bonds with Pu (IV) ion, and hence a real comparison can be made with respect to its interaction with GO. As it can be seen in (Figure 4a), when plutonium nitrate complex approaches type-I GO, water molecules still remain closest to the GO nanosheet, indicating weak interaction between plutonium ion and GO (type I). This observation is similar to the one found for the interaction of plutonium complex with graphene nanosheet.
The MOs also corroborate the fact that −OH and −O− do not causes strong interaction with Pu (IV) complex as HOMO and LUMO of GO (type I) remain unaffected by the presence of Pu (IV) complex. Hence a GO with carbonyl group (type II) was tested as −CO group is known to form strong coordination complexes with d-block elements. As shown in (Figure 4b), −CO group attached to the graphene causes rearrangement of nitrates around Pu (IV) ion to accommodate carbonyl group, indicating strong interaction. The MEPs for two cases are Illustrated in (Figure 4c,d).
Current−voltage characteristics for interaction of plutonium complexes with graphene and GO are shown in (Figure 5a). No appreciable change in I−V characteristics of graphene is observed when plutonium complex approaches a graphene sheet. There is no appreciable change in π-π orbitals on graphene surface in the presence of Pu4+ complex, indicating a weak interaction with graphene and a strong interaction with surrounding nitrate ions.
The conductance is found to increase marginally from 0.67 to 0.93 μAV−1. Similar results are obtained for GO with −O− functional group However, the conductance of GO is found to decrease in this case from 0.007 to 0.006 μAV−1. Hence a GO with a carbonyl group is tested for its interaction with plutonium complexes. As a result, a significant reduction in current values is observed in the presence of plutonium complex (Figure 5c). The conductance of GO in this case reduces significantly from 9.5 to 1.6 μAV−1 in the presence of Pu (NO3) 4(H2O)3 complex. From the above results, we can conclude that GO with –CO group is the most promising sensing material for detection of U/Pu complexes. The change in current−voltage characteristics can be used as a marked signature to identify radio nuclides present in nuclear wastewater.
ConclusionsMEP calculations of U and Pu complexes with graphene reveal that graphene needs to be functionalized to act as an effective sensor for detection of fissile materials. From previous knowledge that GO has high affinity for U and Pu complexes. Changes in the MEP due to the presence of these complexes near graphene or GO can be transduced and amplified into current−voltage characteristics on the nanoscale. By comparing the changes in current, we should be able to detect trace amounts of these radionuclides. Researchers calculations show significant reduction in current values for given bias voltage when plutonium complexes approach GOs containing −CO functional group as compared with a graphene sheet or type-I GO with −O− functional group due to weak interaction. −CO group attached to graphene surface causes nitrates around central Pu(IV) ion to displace, indicating strong interaction.
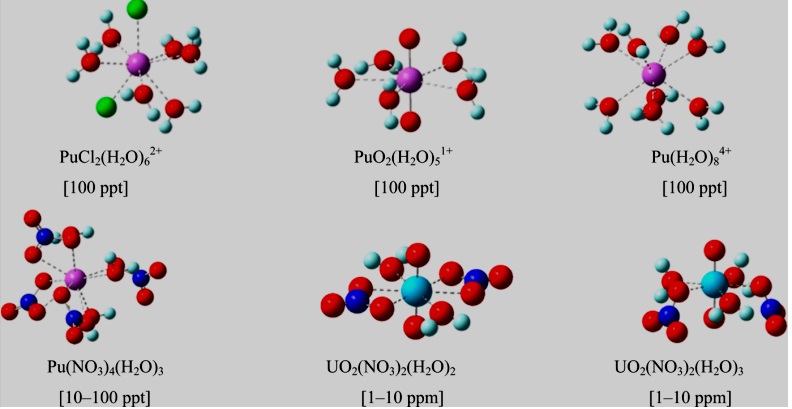
Figure 1: Common U and Pu moieties in nuclear wastewater [12].
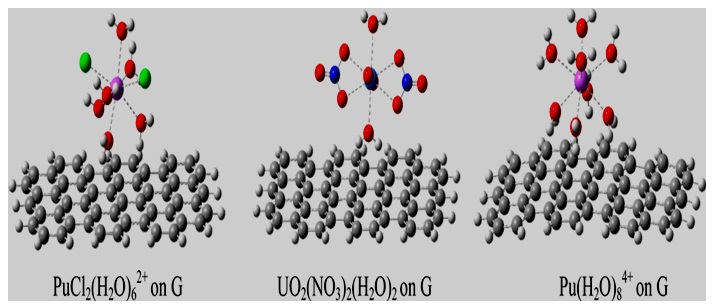
Figure 2: Optimized geometries of some common U and Pu moieties adsorbed on a graphene (G) molecule [12].
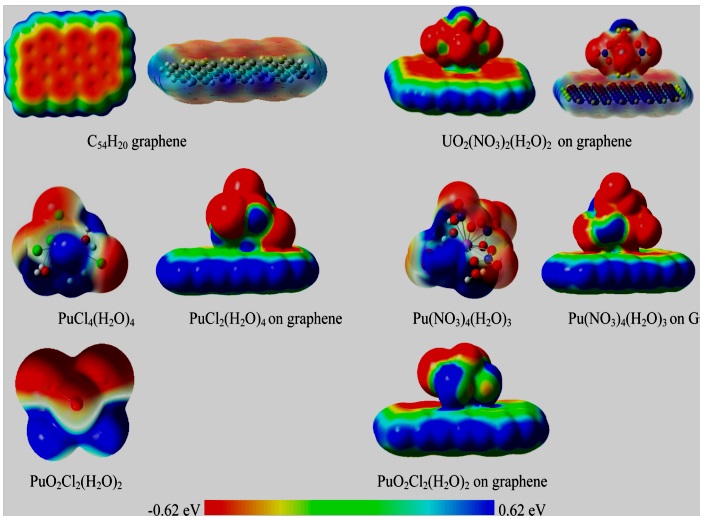
Figure 3: MEPs of graphene adsorption to U and Pu complexes [12].
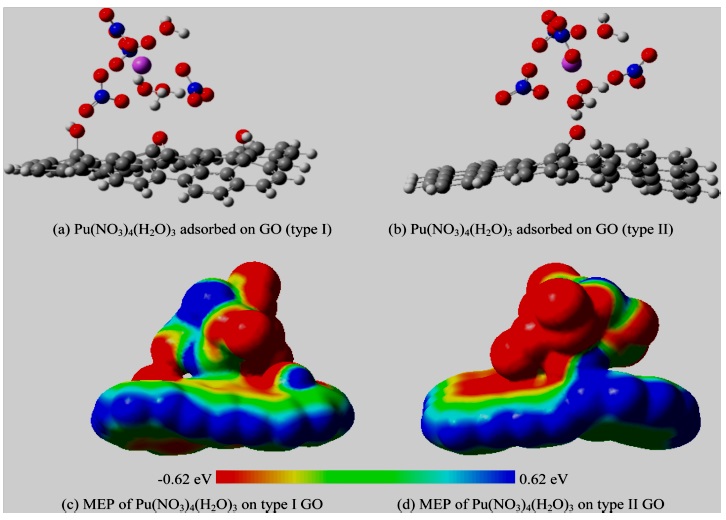
Figure 4: Optimized geometry of nitrato-aquo-complex of Pu (IV) adsorbed on GO (type I) and GO (type II) and their corresponding molecular electrostatic potential (MEP) [12].
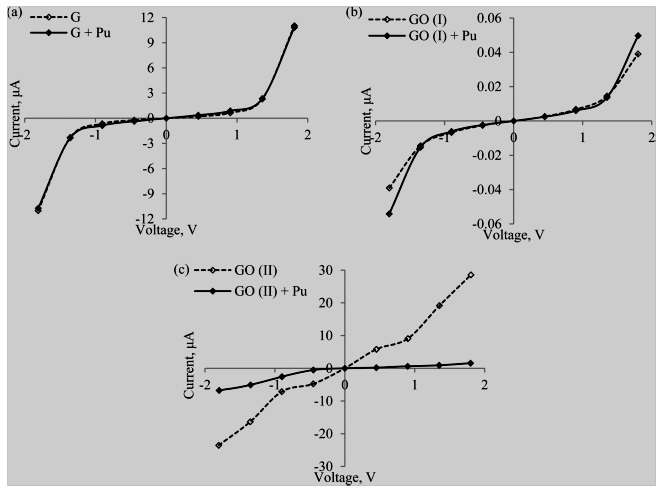
Figure 5: Current−voltage characteristics of Pu(IV) complex adsorbed on graphene/graphene oxide. (a) (NO3)4(H2O)3 on graphene. (b) Pu(NO3) 4(H2O)3 on GO (type I). (c) Pu(NO3)4(H2O)3 on GO (type II) [12].
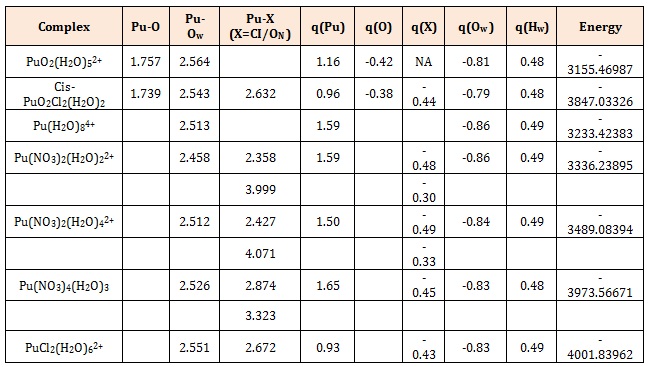
Table 1: Calculated Bond Distances, Atomic Mulliken Charges, and Energies of Pu Complexes Adsorbed on Graphene [12].
Chat with us on WhatsApp