Citation: Judeh ZMA, et al. Recent Advances and Developments in Cochleate Technology. Nanomed Nanotechnol 2017, 2(2): 000119.
*Corresponding author: Zaher MA Judeh, School of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, N1.2 B1-14, Singapore 637459, Tel: (+65)-67906738; Email: zaher@ntu.edu.sg
Cochleates are lipid based cylindrical solid particulates formed by rolling of large continuous bilayer sheets. They are promising drug delivery vehicles with sustained release capabilities and show improved bioavailability, efficacy as well as reduced toxicity of encapsulated drugs. They are known to be stable and hence enhance the quality of drug formulation by increasing the shelf-life of the formulation. Their nano- to sub-micrometer size range allows for delivery through various routes of administration. This review highlights recent advancements in cochleate technology with respect to their composition, self-assembly, formulation and a brief account of their applications.
Keywords: Cochleates; Nanocochleates; Drug delivery; Lipid particles; Liposomes
Cochleates are a system of solid particulates made up of large continuous sheets of lipid bilayers rolled up to form a cylinder with little or no aqueous phase incorporated within its core (Figure 1) [1]. Due to their unique multilamellar structure, biocompatibility and higher stability, cochleates have been investigated as delivery vehicles for various classes of drugs including delivery of antigens and peptides in the form of vaccines [2-4]. There have been several modifications in strategies to prepare cochleates with suitable structures and sizes to prepare a specific application. It was only by the beginning of year 2000 that strategies to form nanocochleates were developed [5-7]. Development of methods such as hydrogel isolation [8], emulsionlyophilization [9], and microfluidics-based microspherical cochleate composites [10] have opened application-based research where cochleates with sizes in the nano- and sub-micrometer range can be obtained.
Composition and Chemistry of CochleatesCochleates essentially consist of negatively charged phospholipids such as phosphatidylserine (PS), dioleoylphosphatidylserine (DOPS), phosphatidylinositol (PI), phosphatidylcholine (PC), phosphatidic acid (PA) and phosphatidyl glycerol (PG) and/or a mixture of one or more of these lipids with other lipids. Rolling of lipid sheets into a cochleate structure is generally facilitated by a divalent cation such as Ca2+, Mg2+, Ba2+, Zn2+ or Fe2+ [11]. Among the divalent cations, Ca2+ has been reported to form more tightly packed structures with less water of hydration and at a lower concentration[12]. Also, it is well reported that Ca2+ plays an important role in natural membrane fusion processes making this part of the cochleate structure biocompatible. Water soluble cationic drugs or peptides such as tobramycin and oligo-acyllysine have also been found to act as inter-bilayer bridges by interacting with the negatively charged lipids instead of divalent cationic metal ions [13,14].
Mechanism of Self-Assembly of CochleatesCochleates are formed by nucleation-dependent continuous aggregation and self-assembly process leading to the growth of cochleate particles. Several theories have been proposed to explain the mechanism of formation of cochleate systems. It is believed that cochleation occurs through several intermediate steps: in the presence of cationic bridging molecules bilayer vesicles are ruptured and undergo fusion to form large planar bilayer stacks inducing development of hydrophobic surfaces which in order to minimize the interaction with water, initiates further aggregation of these stacks to form larger sheets which eventually coil to form cochleate cylinders [15,16]. The presence of several early intermediate structures such as liposome aggregates, disc micelles or ribbons has been recently reported along with higher order intermediate structures like stacks and sheets during characterization of lipids with a low transition temperature (Tm) [17]. Figure 2 shows a possible mechanism of cochleate formation. Techniques like cryogenic electron microscopy (Cryo-TEM, Cryo-SEM and freeze-fracture SEM) have helped in structural characterization of cochleate structures and elucidating the underlining mechanism of their formation [18].
Methods of Preparation and Recent AdvancesOver years several methods have been developed to prepare cochleates. Cochleates essentially are stacked phospholipid bilayers formed by the fusion of small unilamellar vesicles upon addition of positively charged bridging molecules (Ca2+). Hence, the general method to prepare cochleates would be to first obtain uniform liposomes and then expose these spherical vesicles to divalent cations to form cylindrical cochleates. The conventional strategies for the preparation of cochleates include trapping and dialysis methods which are known to form cochleate structures of large and varied particle sizes mainly due to the continuous uncontrolled aggregation of lipid sheets [7].
Trapping methods require formation of liposome suspension followed by addition of divalent cations. The two most common trapping methods are high pH trapping and trapping film methods [6]. Dialysis can be incorporated in two ways to prepare cochleates namely, direct cochleates (DC) dialysis and liposome before cochleates (LC) dialysis methods. DC dialysis method relies on directly dialyzing the mixture of detergent and lipids against a solution containing divalent cations. Whereas, LC dialysis method is a two-step process involving removal of detergents by dialysis to form liposomes first and the liposomes are further dialyzed against a solution of divalent cations to transform them into cochleates [3,6].
Over the last decade, the research has been focused on the development of strategies to produce nanocochleates by restricting the number of building blocks that fuse to form cochleate. Methods such as binary aqueous-aqueous emulsion, hydrogel isolation and emulsificationlyophilization have enabled the formation of more uniform nano-sized cochleates particles [5,8,9]. These methods are based on incompatibility of different immiscible polymers in two-phase systems. However, these methods commonly involve the formation of colloidal structures in a multistep process and often require steps such as handling emulsions, washing polymers, etc., which are complex, tedious, timeconsuming and difficult to scale up. Most recently, Fahr and co-workers introduced technique of nanoprecipitation using microfluidics device for preparation of micro-spherical particles made up of nanocochleate subunits called micro-spherical cochleate composites [10]. This technique uses the combined benefits of nanoprecipitation and microfluidics and therefore can exercise better control over flow rates, mixing etc. while eliminating the use of processes like emulsification.
Existing Applications and Advantages of Cochleate SystemDue to their nature, cochleates are preferably used for hydrophobic and amphipathic drugs as they can be easily inserted into the membrane bilayers. Several nucleotides, peptides and aminoglycosides can also be embedded into cochleates due to their pH-induced charge based properties [7]. Cochleates are considered to be well suited for oral and intramuscular delivery routes due to their rigid structure, unlike liposomes. In addition, cochleates are non-toxic, and considered to have high stability both in solution preserved for long periods at 4°C and as lyophilized powder stored at room temperature prior to administration [11]. These unique attributes provide a real opportunity for cochleates to deliver drugs having low bioavailability.
Nanocochleates have been used for oral delivery of antifungal drug Amphotericin B (AmB) to treat systemic candidiasis with improved oral absorption and anti-fungal activity compared to conventional drug solution. Administration of low doses at 0.5 mg/kg body weight of AmBcochleates (CAMB) completely cleared the fungal infection from kidneys and lungs, with no trace of toxicity. CAMB did not induce hemolysis of human red blood cells even at high concentrations of 500 μg AmB/ml, whereas conventional drug deoxycholate AmB (DAMB) solution was highly hemolytic at 10 μg AmB/ml. Also, cochleates were found to be highly effective in treating mice infected with Candida albicans when administered intraperitoneally at doses as low as 0.1 g/kg/day [19,20]. Recently several studies have incorporated cochleate technology for delivery of insulin and anti-cancer drugs such as paclitaxel, fisetin and raloxifene [2,21,22]. In addition, cochleates are shown to allow active targeting for delivery of drugs using ligand targets or magnetocochleates by encapsulating cochleates with magnetic particles [23,24].
It has been suggested that the membrane fusion capability of cochleates is responsible for their enhanced activity. Studies have reported that, while calcium ions present in cochleates promotes membrane-cochleate interaction; the high tension at the bilayer edges of cochleates act as the driving force for their interaction with tissue membranes [2,20]. When administered orally cochleates are preferentially endocytosed by macrophages in the gastrointestinal tract and thus delivering the drug to the site of action through lymphatic vessels [13,23]. Kellermayer and co-workers reported the extreme mechanical resilience of cochleates due to their tightly packed structure which provides unparalleled protection for the drug molecules to be encapsulated and delivered to the site of action [25]. The non-aqueous nature of cochleates renders them resistant to penetration by oxygen and water making the lipids less susceptible to oxidation or hydrolysis, thus protecting the drugs from the harsh acidic environment and the digestive enzymes in the gastrointestinal tract.
Conclusion and Future ProspectsCochleates have emerged as a promising scaffold for drug delivery with sustained release capability. Owing to their phospholipid framework, they can improve bioavailability, efficacy and reduce the toxicity of incorporated active substances. They are also known to enhance the quality of drug formulation by increasing the stability and shelf-life of the formulation. Therefore, cochleates technology can be applied to photolabile and drugs which get degraded by oxidation. There is scope for further investigations involving new cochleate administration routes. Also, the possibility of delivering drugs by active targeting using ligand targets or encapsulating cochleates with magnetic particles to obtain magneto-cochleates requires further investigations. Considering the fact that drugs formulated as cochleates such as amphotericin B-cochleate have reached clinical trials, cochleates as drug delivery systems are gaining importance in the pharmaceutical industry. Although some of the key issues such as cost, better formulation strategies, the detailed mechanism of action etc. need to be addressed, cochleates definitely provide a strong potential as drug delivery vehicles.
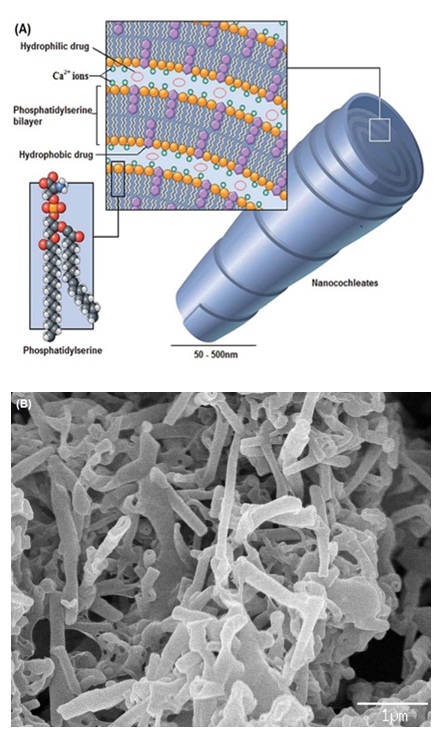
Figure 1: (A) Schematic of nanocochleates (Reproduced from reference [11] with permission from the Royal Society of Chemistry). (B) Scanning electron micrograph of cochleates (Shuddhodana, Nagarsekar K and Judeh ZMA. Unpublished data)
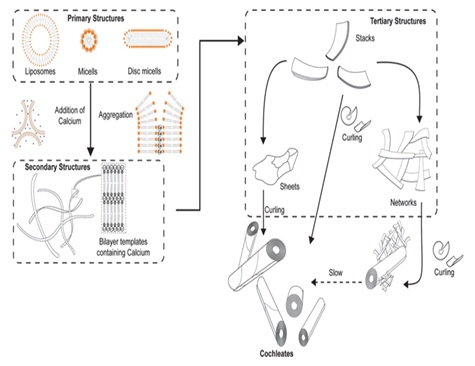
Figure 2: Schematic of possible mechanism of cochleate self-assembly (Reproduced from reference [17] with permission from the Royal Society of Chemistry).
Chat with us on WhatsApp