
Citation: Osuga T, et al. N-Methylacetamide for Cryopreservation - An Alternative to Dimethyl Sulfoxide. Nanomed Nanotechnol 2017, 2(2): 000121.
*Corresponding authors: Toshiaki Osuga, Center for Frontier Medical Engineering, Chiba University, Yayoi, Inage, Chiba
263-8522, Japan, Tel: 043-290-3123, Fax 043-290-3123; Email: artisankoshik@yahoo.co.jp
Takayoshi Asai, Japanese Red Cross Chiba Blood Center, Chuo, Chiba 274-0053, Japan, Tel: 047-457-0711, Fax 047-457-
7304; Email: tasai-th@umin.ac.jp
Cell death due to cryopreservation is caused by insufficient protection from freezing-induced damage and cytotoxicity of the cryopreservation agent, acting both before freezing and after thawing. Compared to dimethyl sulfoxide (DMSO), Nmethylacetamide (NMA) was previously suggested to have lower cytotoxicity in cryopreserved cells because the sulfinyl group, which has high polarity and d-orbital effect, is replaced by an amide group, which has moderate polarity. Furthermore, NMA retains the two methyl groups present in DMSO, which are known to confer permeation ability to DMSO. Therefore, NMA is expected to have lower cytotoxicity than that of DMSO, while retaining both the cryopreservative activity and permeation ability of DMSO. We performed cell viability experiments in cells incubated with DMSO and NMA and then cryopreserved. We found that, after thawing and complete removal of cryopreservatives, cells cryopreserved with NMA exhibited a higher recovery rate than those cryopreserved with DMSO. Moreover, after 1 h of incubation with 5% cryopreservative at room temperature, the cell survival rate was higher for NMA than for DMSO, indicating that NMA has lower cytotoxicity under conditions corresponding to the pre-freezing state and after thawing. No significant differences between NMA and DMSO were observed regarding the colony-forming ability per cell that survived after thawing and complete removal of cryopreservatives. Finally, to achieve similar cryopreservation action (post-thawing recovery rate, 75.5%), the necessary NMA concentration was estimated to be lower than the DMSO concentration (3% vs. 5%). Our findings support the use of NMA for cryopreservation.
Keywords: Hematopoietic Stem Cell; Cryoprotectant; Freezing-Induced Damage; Cytotoxicity
Dimethyl sulfoxide (DMSO) [H3C-SO-CH3] (Figure 1), which consists of one sulfinyl group [-S(O)-] bound to two methyl groups [-CH3], is used as an osmotically active agent for cryopreservation of living cells. DMSO acts as a scavenger of radicals generated by inflammation and radiation [1,2]. Oxidation of luciferin for bioluminescence assays is carried out with DMSO [3], and the melting temperature of DNA (thermal denaturation of DNA) can be lowered by increasing the concentration of DMSO [4]. However, the d-orbital effect, oxidation reactivity, and high-polarity of the sulfinyl group in DMSO often lead to cytotoxicity [1]. Replacement of the sulfinyl group [-S(O)-] in DMSO with an amide group [-C(O)-N(H)-] yields Nmethylacetamide (NMA) [H3C-CO-NH-CH3] (Figure 1). Because the amide group has moderately high polarity and is naturally involved in bioprocesses [5], the cytotoxicity of NMA is lower than that of DMSO in cryopreserved human cells [6].
It was shown that murine erythroleukemia cells cultured in a medium with organic polar molecules such as DMSO or NMA could be successfully induced to differentiate to erythroid cells [7,8]. Because the high permeation ability of DMSO is associated with its two methyl groups, which are also present in NMA, it is expected that NMA will also be able to pass through the cell membranes and prevent formation of ice crystals within the cell. In fact, compared to DMSO, NMA was found to have higher permeation ability for the membrane of sperm cells, and thus is used in the cryopreservation of bovine spermatozoa [9] and fowl semen [10,11]. The aim of the present study was to investigate the efficiency of NMA in the cryopreservation of human cells, as well as to determine the cytotoxicity and side effects associated with the use of NMA as a cryopreservative.
Materials and MethodsThe 100-mℓ cryopreservation solution CP-1 (Kyokuto, Tokyo, Japan), which was used for cryopreservation of umbilical cord blood, contained water with 0.9% (w/v) NaCℓ, 10% (w/v) DMSO, 8% (w/v) human serum albumin (HSA), and 12% (w/v) hydroxyethyl starch (HES) as plasma components for oncotic pressure adjustment [12]. The collected umbilical cord blood (100 mℓ) was mixed with CP-1. The final concentrations of the cryopreservation agents in the equi-volume mixture of blood and CP-1 (200 mℓ) were 5% (w/v) DMSO, 4% (w/v) HSA, and 6% (w/v) HES. The concentration of HSA was adjusted to 4% (w/v) to imitate the HSA concentration normally found in the blood. The umbilical cord blood was cryopreserved in CP-1 at -80°C.
Alternative cryopreservation solutions were obtained by replacing the DMSO in CP-1 with other polar molecules. While glycerol [C3H5 (OH) 3] can cryopreserve erythrocytes and has low cytotoxicity, it cannot cryopreserve leukocytes and thus was not a candidate for the alternative cryopreservative in our experiments. On the other hand, NMA and N-methylformamide (NMF) [HCO-NH-CH3] (Figure 1) both contain one amide group bound to two and one methyl groups, respectively.
Moreover, DMSO, NMA, and NMF were previously shown to allow erythropoietic differentiation [7,8]. Therefore, alternative cryopreservation solutions containing NMA or NMF were obtained, and the cryopreservation action was compared. The cryopreservation solutions contained NaCℓ, HSA, and HES in concentrations similar to those in CP-1.
The cryopreservation ability and cytotoxicity of solutions containing DMSO, NMA, and NMF were compared to determine the suitability of these compounds as cryopreservation agents. All cryopreservation experiments were conducted in the same way. The cell solutions were obtained by incubating human HL-60 and RPMI8226 cells, as well as mouse bone marrow (BM) cells in cryotubes (diameter, 10.0 mm) filled with the cryopreservation solution. The cells were cryopreserved at a cooling rate of - 1.0°C/min (freezing rate) [12], incubated at -80°C (freezing temperature) for seven days (freezing time), and then thawed in a water bath at 37°C (thawing temperature) for 20 s (thawing time).
Removal of Cryopreservatives after ThawingThe upper limit of the cryopreservative concentration is determined in consideration of the osmotic pressure acting before freezing and after thawing. The molecular weights of DMSO and NMA are similar (78.1 and 73.1, respectively). The weight concentrations of 5% (w/v) DMSO and 5% (w/v) NMA correspond to 0.640 mol/ℓ and 0.684 mol/ℓ, respectively. The optimal concentration of DMSO and NMA for cryopreservation is >0.6 mol/ℓ, which is four-fold higher than the molar concentration of saline (0.154 mol/ℓ, corresponding to 0.9% (w/v) NaCℓ). Therefore, the contact between the cryopreservation solution and living cells should be minimized before freezing and after thawing. Indeed, residual DMSO after thawing generates side effects after cell transplantation [1,2]. Such effects were also noted for residual glycerol. For example, when cryopreserving fowl semen with glycerol, the residual concentration of glycerol after thawing must be below 0.163 mol/ℓ prior to artificial insemination to avoid the contraceptive effect of glycerol [10].
Therefore, after thawing and before conducting cell viability assays, we performed complete removal of the cryopreservatives following a consistent protocol. Specifically, after thawing, the cell solution was stirred and transferred into a centrifuge tube of 15 mℓ, which was filled with a new saline solution. The centrifuge tube was stirred and then centrifuged at 250 g for ten minutes, allowing the cells to precipitated at the bottom of the centrifuge tube. After the supernatant was discarded by decantation, the centrifuge tube was filled with new saline solution and stirred in order for the cells at the bottom to distribute into the entire volume of this new cell solution. Because the supernatant volume was greater than 0.95 of the entire solution, the residual ratio was less than 0.05. These processes of centrifugation, decanting, dilution, and stirring were repeated three times, aiming for a final residual concentration of the cryopreservative in the cell solution of <10-3 of the concentration of the cryopreservative before freezing, which is considered complete removal. The final cell solutions (i.e., after removal of DMSO, NMA, HSA, and HES) were used for viability assays using trypan blue, to determine the cell recovery rate.
Viability and Colony Assay to Evaluate Cryopreservative Action and CytotoxicityThe post-thawing recovery rate of cryopreserved cells is mainly related to the efficiency of the cryopreservative in protecting against freezing-induced damage due to formation of ice crystals inside the cells. Additionally, post-thawing cell recovery is influenced by the cytotoxicity of the cryopreservative solution acting on cells before freezing and after thawing. For this reason, we evaluated the cytotoxicity of the cryopreservation solutions at room temperature. Specifically, the cells were placed in saline solution and incubated with DMSO or NMA solutions at room temperature for up to 5 h. The viability assay using trypan blue was performed at 1-h intervals from time zero hour until five hours later. We thus obtained the decay curve for cell viability, based on which we could evaluate the cytotoxicity caused by the cryopreservation solutions.
After thawing and complete removal of the cryopreservatives, a colony assay for mouse BM cells was performed, whereby the stem cells were induced to differentiate into leukocytes and erythrocytes, to determine whether the cryopreservative affects the colony-forming ability for cryopreserved cells that survive after thawing and complete removal of the cryopreservative.
ResultsWe prepared three cryopreservation solutions containing 0.9% (w/v) NaCℓ, 4% (w/v) HSA, and 6% (w/v) HES in water in three dishes, and added a different polar molecule to each, namely 10% DMSO, 10% NMA, and 10% NMF. Human HL-60 cells and human RPMI8226 cells were cryopreserved with each of these three cryopreservation solutions at -80°C. After seven days, the cells were thawed. The recovery rates of HL-60 and RPMI8226 cells are shown in Figures 2(A) and (B), respectively. The recovery rates of HL-60 cells after cryopreservation using DMSO, NMF, and NMA were 91.0%, 22.5%, and 92.0%, respectively. For RPMI8226 cells, the recovery rates after cryopreservation using DMSO, NMF, and NMA were 84.0%, 27.0%, and 85.0%, respectively. In both cell lines, cryopreservation using DMSO or NMA resulted in high recovery rates (≥84%), compared to <30% after cryopreservation using NMF. Therefore, although NMA and NMF both contain the amide group, the significantly lower cell recovery rate noted after cryopreservation with NMF suggests that two methyl groups are required. Therefore, we further compared only the cryopreservation actions of DMSO and NMA, which contain two methyl groups.
We prepared nine cryopreservative solutions containing 4% (w/v) HSA and 6% (w/v) HES in saline in nine dishes. To one dish, we added 5% DMSO; to the other eight dishes, we added NMA at concentrations from 1% to 8% (w/v), with 1% (w/v) increments, to determine the optimum concentration of NMA for cryopreservation. We added human RPMI8226 cells to the nine dishes and cryopreserved them at -80°C. After seven days, we thawed the cells and measured recovery rates (Figure 3). The use of 5% DMSO yielded a 75.4% recovery rate after thawing. The recovery rate of RPMI8226 cells was 12.2% at 1% NMA, increased to 33.9% at 2% NMA, further increased to 75.5% at 3% NMA, but remained relatively constant, with 82.0%, 79.2%, 77.4%, 74.5%, and 75.5% at 4%, 5%, 6%, 7%, and 8% NMA, respectively. We noted an average cell recovery rate of 77.3% for NMA concentrations above 2%, with a minimum of 74.5% for NMA concentrations of 3%–8%. Therefore, in the following analysis, we compared the cryopreservation action of DMSO and NMA at 5% weight concentration.
Cytotoxicity of Cryopreservatives before Freezing and after ThawingWe prepared two cryopreservation solutions containing 4% HSA and 6% HES in saline in two dishes. We added 5% DMSO to one dish and 5% NMA to the other. We then added human RPMI8226 cells to the two dishes and incubated them at room temperature (22°C). To evaluate the cytotoxicity of DMSO and NMA, we compared the cell survival rates at 1-h intervals from time zero to 5 h of incubation (Figure 4). After removing the cryopreservatives (DMSO, NMA, HSA, and HES) by repeated centrifugation and dilution with saline, we performed cell viability assays using trypan blue. We found that the cryopreservation solution containing NMA maintained the survival rate above 96% throughout the entire 5 h of incubation, whereas the survival rate of cells incubated with cryopreservation solution containing DMSO decreased to below 96% within the first 2 h, reaching 93% at the end of the 5-h incubation period (Figure 4). Since the decrease in cell survival rate following incubation with cryopreservation solution is likely related to the cytotoxicity of the cryopreservation agent, it is considered that the higher cell survival noted for NMA indicates lower toxicity compared to that of DMSO.
Colony-forming Ability Per Cell that Survived after ThawingWe prepared two cryopreservation solutions containing 4% HSA and 6% HES in saline in two dishes. We added 5% DMSO to one dish and 5% NMA to the other. We then added mouse BM cells to these dishes and cryopreserved them for seven days. After thawing the cells, we evaluated the recovery rates (Figure 5). We defined the viability ratio BMVr (NMA/DMSO) = 1.03 as the recovery rate of mouse BM cells cryopreserved with NMA (70.0%) divided by the recovery rate of cells cryopreserved with DMSO (68.0%).
After performing the viability assay and removing the cryopreservatives, we placed the mouse BM cells in the culture media M3434 (Methocult), which is optimized for the growth and enumeration of hematopoietic progenitor cells in colony-forming unit (CFU) assays of mouse BM cells. After two weeks, we thawed the cells and assessed the colony-forming abilities of the cells cryopreserved with DMSO and NMA (Figure 6). We evaluated colonyforming ability in terms of CFU-GM and CFU-GEMM, which refer to granulocyte-macrophage CFUs and granulocyte- erythrocyte-monocyte-megakaryocyte CFUs, respectively. Furthermore, we evaluated CFUGM(NMA/DMSO) and CFU-GEMM(NMA/DMSO), which describe the colony-forming ability of cells cryopreserved with NMA relative to the ability of cells cryopreserved with DMSO (colony forming ability ratio).
We found the colony-forming ability ratios to be BMCFUGM (NMA/DMSO) = 1.25 and BMCFU-GEMM(NMA/DMSO) = 1.08 (Figure 6), where the superscript BM indicates mouse BM cells. Since all cells were placed on cultivation dishes regardless of whether they were alive or dead after thawing, the colony-forming ability ratios are affected by the viability ratio BMCFU (NMA/DMSO) = 1.03 determined immediately after thawing and complete removal of the cryopreservatives. By dividing the colony-forming ability ratio by the viability ratio, we obtained the colonyforming ability per cell that survives after thawing as BMsCFU-GM(NMA/DMSO) = BMCFU -GM(NMA/DMSO) / BMVr (NMA/DMSO), where the subscript s means per surviving cell (rectangles marked in dotted lines in Figure 6).
Thus, we calculated the colony-forming ability ratios of as BMsCFU-GM(NMA/DMSO) = 1.21 and BMsCFUGEMM(NMA/DMSO) = 1.05, based on which we could compare the effect of NMA and DMSO on the colonyforming ability per cell that survives after thawing. Although the colony-forming ability (CFU-GM and CFUGEMM) of mouse BM cells cryopreserved with NMA were higher than that of cells cryopreserved with DMSO, there is no conclusive basis for choosing DMSO or NMA for mouse BM cell cultivation assuming complete removal of the cryopreservative prior to cultivation. Our present findings indicate that, compared to using DMSO, using NMA as a cryopreservative is likely to result in higher cryopreservation action, lower cytotoxicity, and similar colony-forming ability after complete removal of the cryopreservative.
DiscussionThere have been significant advances in treatment modalities for hematologic malignancies, such as the widely-applied transplantation of cord blood and peripheral blood stem cells. Concomitant with these advances, safe and effective stem cell preservation techniques should be developed to facilitate further advances in the medical field. Cryopreservatives are used to minimize freezing-induced cell damage, as they prevent the growth of ice crystals by interfering with the polarized water arrangement during ice nucleation. Although DMSO can protect living cells against freezing-induced damage, the sulfinyl group in DMSO often induces cytotoxicity. Therefore, it is necessary to identify a cryopreservation agent that does not contain the sulfinyl group and has lower cytotoxicity.
Similar to the behavior of transition metals, the sulfur atom can adopt various valence states owing to the existence of unoccupied d-orbitals (d-orbital effect). In living systems, DMSO is metabolized to dimethyl sulfide [H3C-S-CH3] (Figure 1) with release of reactive oxygen species, which leads to cytotoxicity and side effects in transplanted cells. In NMA, the sulfinyl group is replaced by an amide group, which has moderate polarity and does not exhibit d-orbital effect, contributing to the lower cytotoxicity of NMA relative to that of DMSO. Furthermore, like DMSO, NMA also contains two methyl groups, which is known to improve the permeation ability, allowing DMSO to pass through cell membranes and prevent formation of ice crystals within the cell. Therefore, it is expected that cryopreservation with NMA results in lower cytotoxicity without compromising cryopreservation activity or permeation ability. Our present results confirmed this hypothesis.
Like NMA, NMF also contains the amide group instead of the sulfinyl group, but further contains only one methyl group (the other being replaced by hydrogen). Despite the similarity between NMA and NMF in terms of the amide group, we found that the recovery rate of human RPMI8226 cells cryopreserved with 10% (w/v) NMF was significantly lower than that of cells cryopreserved with 10% (w/v) NMA or 10% (w/v) DMSO, suggesting that both methyl groups are required to achieve sufficient cryopreservation activity.
We found that, after thawing and complete removal of the cryopreservative; cells cryopreserved with NMA exhibited a higher recovery rate than cells cryopreserved with DMSO. Cell death due to cryopreservation is influenced by two factors, namely protection from freezing-induced damage and cryopreservative-induced cytotoxicity acting before freezing and after thawing. Therefore, to identify suitable candidates for cryopreservation agents, both the cryopreservation action and the cytotoxic effect should be assessed. We found that cells incubated at room temperature for 5 h maintained a higher survival rate in the presence of 5% (w/v) NMA solution than in the presence of 5% (w/v) DMSO solution, indicating that NMA has lower cytotoxic effect before freezing. Furthermore, these results suggest that the cytotoxic effect of NMA will be less pronounced than that of DMSO if complete removal of the cryopreservative is not achieved after thawing, which is expected to happen in the clinical setting. Therefore, using NMA rather than DMSO is expected to attenuate cell death due to cryopreservation, both by ensuring sufficient cryopreservation action and by reducing cytotoxicity. Additionally, the use of NMA is expected to reduce side effects occurring after hematopoietic stem cell transplantation assuming incomplete removal of the cryopreservative after thawing.
We conducted colony assays to evaluate the effects of DMSO and NMA on the colony-forming ability per cell that survived after thawing assuming complete removal of the cryopreservative. It is important to note that the overall recovery rate after thawing was re-evaluated as per cell that survived because DMSO and NMA provided different cell viability rates after thawing, which necessarily influenced the number of CFUs since all thawed cells (i.e., dead or alive) were placed on cultivation dishes. No significant differences were observed in the colonyforming ability per cell that survived after thawing the cells cryopreserved with DMSO and NMA and complete removal of DMSO and NMA.
It was previously reported that the lowest concentration of NMA and DMSO required for erythropoiesis induction in murine erythroleukemia cells was 5 mmol/ℓ and 70 mmol/ℓ, respectively [7,8]. Since DMSO and NMA are added at molar concentrations of 640 and 684 mmol/ℓ, respectively, it is important to ensure that, after thawing and cryopreservative removal, the residual concentrations are sufficiently low. Our protocol for cryopreservative removal ensured that the residual concentration of the cryopreservative in the final cell solution was below 10-3 of the original concentration (i.e., before freezing), i.e., <0.7 mmol/ℓ, which is well below the reported limits for induction of cell differentiation. Thus, it is highly unlikely that residual DMSO and NMA induced abnormal differentiation of the mouse BM cells during the colony assay.
At 5% (w/v), which is the minimum concentration necessary for achieving sufficient cryopreservation activity with DMSO, the molar concentrations of NMA (640 mmol/ℓ) and DMSO (684 mmol/ℓ) are four times higher than that of saline (154 mmol/ℓ). Therefore, it is desirable to identify a cryopreservative that can provide similar cryopreservation activity at lower concentrations. In our experiments, we found that post-thawing recovery rates were higher in cells cryopreserved with 3% (w/v) NMA than in cells cryopreserved with 5% (w/v) DMSO (82.0% vs. 75.4%), indicating that NMA may indeed be a suitable alternative to DMSO. However, further investigation is warranted to determine the optimal NMA concentration for achieving sufficient cryopreservation action and high cell recovery rates.
ConclusionThe establishment of safe and effective stem cell cryopreservation techniques is necessary to improve medical treatment. In agreement with previous reports, our present findings confirmed the effectiveness of NMA as a cryopreservative in human and mouse cells, as well as its lower cytotoxicity compared to that of DMSO. Therefore, NMA represents a promising alternative to DMSO for the cryopreservation of living cells.
AcknowledgementsT.O. is grateful to the late Dr. Isao Kamiya, Professor Emeritus of Nagoya University for his insightful suggestions. We are grateful to Mrs. Megumi Naito for technical support. This study was partly supported by a Grant-in-Aid from Japan Society for the Promotion of Science (No. 22850114).
DisclosureThe authors declare no conflict of interests.
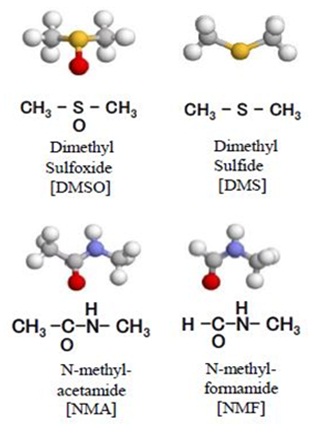
Figure 1: Chemical structure of (A) DMSO, (B) DMS, (C) NMA, and (D) NMF. Models created using RasMol. The high-polarity sulfinyl group [-S (O)-] in DMSO, as well as the interconversion between DMSO and DMS may lead to cytotoxicity.
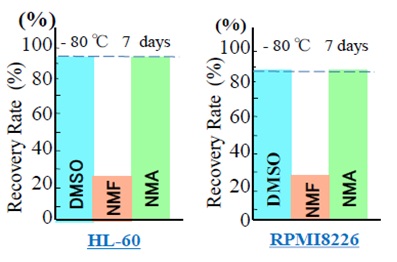
Figure 2: Post-thawing recovery rates of cells cryopreserved with DMSO, NMF, or NMA. The cryopreservatives were added at 10% (w/v) before freezing, and were removed before the viability assay. (A) HL-60 cells. (B) RPMI8226 cells. DMSO: Dimethyl sulfoxide; NMA: N-methylacetamide; NMF: N-methylformamide.
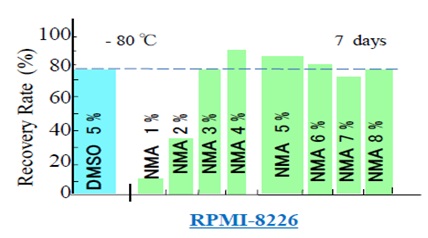
Figure 3: Determination of the optimum concentration of NMA as a cryopreservative. After thawing, RPMI-8226 cells exhibit a recovery rate of over 75% at NMA concentrations ranging from 3% to 8% (w/v), compared to recovery rates of 12.2 % and 33.9% at 1% and 2% (w/v) NMA, respectively. The post-thawing recovery rate was similar (75.5%) for RPMI-8226 cells cryopreserved with 3% (w/v) NMA and RPMI-8226 cells cryopreserved with 5% (w/v) DMSO. DMSO, dimethyl sulfoxide; NMA, N-methylacetamide
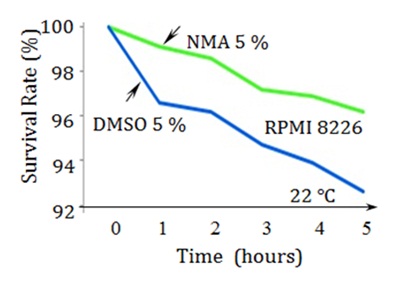
Figure 4: Survival rate of RPMI8226 cells over the course of 5 h of incubation with cryopreservative solution at room temperature (22°C). RPMI8226 cells incubated with NMA showed a survival rate of 96%, compared to under 93% for RPMI8226 cells incubated with DMSO. These measurement conditions correspond to the pre-freezing state after adding the cryopreservative, as well as to the post-thawing state with incomplete removal of the cryopreservative. DMSO: dimethyl sulfoxide; NMA: N-methylacetamide.
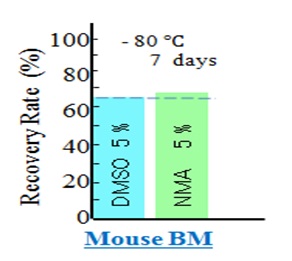
Figure 5: Post-thawing recovery rates of mouse BM cells cryopreserved with DMSO and NMA. After cryopreservation at -80°C, the cell recovery rates were 68.0% and 70.0% for DMSO and NMA, respectively. DMSO, dimethyl sulfoxide; NMA, N-methylacetamide
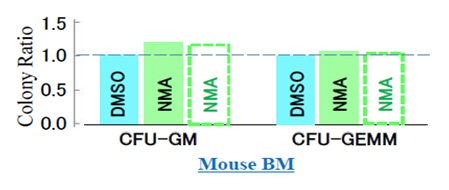
Figure 6: Post-thawing colony-forming ability of mouse BM cells cryopreserved with DMSO and NMA. CFU-GM and CFU-GEMM should be compared on the basis of cell recovery rates because all cells were placed on cultivation dishes, regardless of whether they were alive or dead after thawing. Rectangles marked by dotted lines indicate the colony-forming ability re-evaluated per cell that survived after thawing and complete removal of the cryopreservative. CFU-GEMM: Colony-forming unitgranulocyte, erythrocyte, monocyte/macrophage, megakaryocyte; CFU-GM: Colony-forming unitgranulocyte macrophage.
Chat with us on WhatsApp