
Citation: Nikolelis PD, et al. A Nanosensor for the Rapid Detection of Cholera Toxin Using Graphene Electrodes with Incorporated Polymer Lipid Films. Nanomed Nanotechnol 2018, 3(2): 000137.
*Corresponding author: Dimitrios P Nikolelis, Department Laboratory of Environmental Chemistry, Department of Chemistry, University of Athens, Panepistimiopolis-Kouponia, GR-15771 Athens, Greece, Email:dnikolel@chem.uoa.gr
The present work describes a miniaturized potentiometric cholera toxin sensor on graphene nanosheets with incorporated lipid films. Ganglioside GM1, the natural cholera toxin receptor, immobilized on the stabilized lipid films, provided adequate selectivity for detection over a wide range of toxin concentrations, fast response time of ca. 5 min, and detection limit of 1 nM. The proposed sensor is easy to construct and exhibits good reproducibility, reusability, selectivity, long shelf life and high sensitivity of ca. 60 mV/decade of toxin concentration.
Keywords: Stabilized lipid films; Cholera toxin biosensor; Graphene electrodes; Gangliosides
Abbreviations:CT: Cholera Toxin; DPPC: Dipalmitoyl phosphatidylcholine; NMP: N-methyl-pyrrolidone; CFTR: Cystic Fibrosis Transmembrane conductance Regulator; BLMs: Bilayer Lipid Membranes.
Cholera toxin (CT) is a bacterial polypeptide belonging to a diverse group of biologically active substances that interact with specific gangliosides in natural and artificial membranes. It is produced by the bacterium Vibrio cholera, can cause an epidemic disease leading to rapid dehydration, acidosis, and death within a few hours of exposure.
According to WHO Global Health Observatory data, cholera outbreaks may sum up to 4.3 million morbidity cases and 142.000 deaths per year worldwide, with high rates, though, in Africa and India. Since CT fits the bioterrorism profile, there has been increasing interest in the development of rapid and sensitive methods for its detection [1].
Cholera toxin acts by the following mechanism: First, the B subunit ring of the cholera toxin binds to GM1 gangliosides on the surface of target cells. The B subunit can also bind to cells lacking GM1. The toxin then most likely binds to other types of glycans, such as Lewis Y and Lewis X, attached to proteins instead of lipids [2-4].
Once bound, the entire toxin complex is endocytosed by the cell and the cholera toxin A1 (CTA1) chain is released by the reduction of a disulfide bridge. The endosome is moved to the Golgi apparatus, where the A1 protein is recognized by the endoplasmic reticulum chaperon, protein disulfide isomerase. The A1 chain is then unfolded and delivered to the membrane, where Ero1 triggers the release of the A1 protein by oxidation of protein disulfide isomerase complex [5]. As the A1 protein moves from the ER into the cytoplasm by the Sec61 channel, it refolds and avoids deactivation as a result of ubiquitination.
CTA1 is then free to bind with a human partner protein called ADP-ribosylation factor 6 (Arf6); binding to Arf6 drives a change in the shape of CTA1 which exposes its active site and enables its catalytic activity [6]. The CTA1 fragment catalyses ADP- ribosylation of the Gs alpha subunit (Gαs) proteins using NAD. The ADP-ribosylation causes the Gαs subunit to lose its catalytic activity of inactivating GTP by hydrolyzing it to GDP + Pi, effectively increasing GTP concentration as less is being converted back to inactive GDP. Increased Gαs activation leads to increased adenylate cyclase activity, which increases the intracellular concentration of 3',5'-cyclic AMP (cAMP) to more than 100-fold over normal and over-activates cytosolic PKA. These active PKA then phosphorylate the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel proteins, which leads to ATPmediated efflux of chloride ions and leads to secretion of H2O, Na+, K+, and HCO3 − into the intestinal lumen. In addition, the entry of Na+ and consequently the entry of water into enterocytes are diminished. The combined effects result in rapid fluid loss from the intestine, up to 2 liters per hour, leading to severe dehydration and other factors associated with cholera, including a rice-water stool [7].
The pertussis toxin (also an AB5 protein) produced by Bordetella pertussis acts in a similar manner with the exception that it ADP-ribosylates the Gαi subunit, rendering it unable to inhibit cAMP production [8].
The present pazper describes a nanosensor for the potentiometric rapid detection of cholera toxin based on graphene nanosheets with incorporated lipid films. Ganglioside GM1, the natural cholera toxin receptor, was immobilized on the stabilized lipid films and provided adequate selectivity for detection of this toxin over a wide range of concentrations, fast response time of ca. 5 min, and low detection limit of 1 nM. The proposed sensor is easy to construct, exhibits good reproducibility, reusability, selectivity, long shelf life and high sensitivity of ca. 60 mV/decade of toxin concentration.
Experimental DetailsDipalmitoyl phosphatidylcholine (DPPC) was purchased from Sigma Chemical Co., St. Louis, MO, USA. DPPC was used as lipid for the formation of the films supported on a polymer. The functional monomer, methacrylic acid, and the crosslinker, ethylene glycol dimethacrylate, were both supplied by Aldrich (AldrichChemie, Steinheim, Germany). The initiator, 2, 2’-azobis- (2-methylpropionitrile) (AIBN), was supplied by Merck KgaA (Darmstadt, Germany). Cholera toxin (CT) was purchased from Calbiochem (La Jolla, CA) and GM1 was obtained from FIDIA Research Laboratory (Abano Terme, Italy).
Water was purified by passage through a Milli-Q cartridge filtering system (Milli-Q, Millipore, El Paso, TX, USA) and had minimum resistivity of 18 MWcm. All other chemicals were of analytical-reagent grade. The filters and (nominal) pore sizes used were glass microfiber (0.7 and 1.0 mM, Whatmam Scientific Ltd., Kent, UK).
The stock solution of cholera toxin in methanol was 1.0 mM. Dilute aqueous solutions of cholera toxin were prepared daily just before use. Buffer solutions (PBS) were prepared by mixing appropriate volumes of 0.5 M KH2PO4 and 0.5 M Na2HPO4. All experiments were performed at 25±1o (Figure 1).
The graphene electrode has been prepared and a homogeneous graphene dispersion (ca. 0.4 mg/mL) has been obtained in N-methyl-pyrrolidone (NMP) through mild sonication (using a Bandelin SONOREX Digital 10P sonicator, Sigma-Aldrich, Taufkirchen Germany) for 180 hours and centrifugation at 700 rpm for 2 h [9]. It has been noticed that this increased sonication time is required because the size of the flakes is severely reduced [10,11] which is a critical parameter for several applications; in addition to the reduced flake size, long sonication of graphite can also affect the quality of graphene (i.e., number and position of broken conjugation areas in graphene, so-called graphene atomic- or pointdefects, which can affect the electronic properties of graphene; these defects are predominantly located at the edges of the graphene flakes, and the basal plane of the flakes is relatively defect free.
This graphene suspension has been poured onto a copper wire (d=0.25 mm) mounted on a glass fiber filter; the evaporation of the organic solvent was carried out using a fan heater. This copper wire has been utilized to establish the connection for the extraction of voltage signals for the calibration curve.
Stabilized lipid films were prepared by polymerization, that could take place either by using UV irradiation or thermal polymerization; 5 mg of DPPC were added to a mixture of 0.070 mL of methylacrylic acid, 0.8 mL of ethylene glycol dimethacrylate, 8 mg of 2, 2Ï-azobis-(2- methylpropionitrile), 1.0 mL of acetonitrile. The mixture was spurged with nitrogen for about 1 min and sonicated for 30 min. For the preparation of the stabilized lipid films, 0.15 mL of this mixture was spread on the microfilter. The filter with the mixture was then irradiated using the UV deuterium lamp.
Polymerization was completed within 4 hours. Alternatively, the polymerization could take place by thermal polymerization in 80°C, but the time of polymerization is longer. These membranes were stable to store in air for periods of more than two months. GM1 was incorporated in bilayer lipid membranes (BLMs) during polymerization by spreading 10 mL of the receptor suspension along with the polymerization mixture (i.e., for the preparation of the GM1-incorporated lipid films, the microfilter was spread with 0.15 mL of the polymerization mixture and 10 mL of receptor suspension). These electrodes can be used once (and then disposed of) or repetitively (after regenerated); when not in use, they are kept at 48°C.
The preparation of the potentiometric biosensor concluded after the encapsulation of the filter-supported polymerized lipid film onto the copper wire containing graphene nanosheets. The bioelectrode can be stored at 48°C when not in use; it remains stable for over three months.
Results and DiscussionThe preparation of stabilized in the air lipid films for repetitive uses has been reported in literature [12-14] using thermal polymerization 60–80oC. These high temperature profiles prohibited the incorporation of proteinaceous moieties into the lipid film during the polymerization process; to avoid deactivation, enzymes or receptors could be incorporated afterwards. UV polymerization used in the current study permitted the incorporation of the receptor during film construction, allowing, thereby, optimizing receptor loading.
Previous studies with Raman spectroscopy have provided information on the mechanism of polymerization and how the lipid film is attached to the polymer [15]. The lipid is attached to the polymer through electrostatic bonding. A peak at 1690 cm-1(corresponding to the C=O stretching of the methacrylate) was decreased with time, showing that the C=O bond is altered to C-O-; this signifies the formation of electrostatic bonding between the C-O- and -NHR3+ of phosphatidylcholine.
There was also a shift of the 1176 cm-1 peak to 1195 cm-1 that showed a strong electrostatic interaction between those two groups. These forces can preserve the film, in structure and function, for multiple uses after storage in air; noticeably, air stabilized films exhibit responses similar to freely-suspended BLMs and can be considered as adequate analogues [16].
Graphene nanosheets used herein allowed sensor miniaturization in a facile manner. The electrochemical response of the biosensor has been measured as the potential difference between the working electrode and the reference electrode. A highly stable output has been extracted along with a good linear sensitivity curve over a large dynamic range from 10×10-9 M to 10×10-6 M at pH 7.0; the coefficient of determination, r2, was found to be 0.9992 (n=30). The detection limit, based on the lowest concentration that could be reliably (S/N=3) measured, was 1 nM. The sensor exhibited an excellent sensitivity slope curve with the value of ~60 mV per decade confirming the enhancement in the potential value near the surface of the biosensor as the concentration of the analyte increases.
The high sensitivity achieved can be attributed to the high surface to volume ratio of graphene nanosheets. The variability of the measurement, as relative standard error (5.95% confidence limit), was found to be œ3.8–4.4%, (n=40, on the same sensor, analyst and day) and 4.6–5.2% (n=30, on different days with newly constructed sensors and different analysts). Preliminary investigations on the response of the sensor to CT in the absence of a receptor, indicated a poor output with a sensitivity slope of ~3 mV per decade of concentration, over a concentration range of 1×10-6 M to 1×10-3 M (at pH=7). Thus, it is evident that non-specific binding is not a critical issue in the reliability of measurements of the sensor developed. Recent studies in the association-dissociation kinetics of cholera toxin binding to GM1 revealed that high GM1 concentrations lead to slow CT association kinetics [17]; total internal reflection fluorescence and atomic force microscopy in supported phospholipid membranes [18] directly showed that high molar GM1 densities inhibit CT binding due to ganglioside clustering at the films surface Figure 2.
Thus, the variation of GM1 density on the sensor was considered in this work as a signal optimization option. Various amounts of GM1 were tested up to 10% w/w of lipid concentration. This largest signal recorded was achieved for ratios of lipid toGM1 of 20:1, i.e., 5% w/w GM1 to lipid concentration; nonetheless, larger amounts of GM1 have caused a slight instability of the lipid film. The response times recorded herein (i.e., 5 min) are in good agreement with values reported in literature [19- 21], whereas optimum pH values for CT-GM1 binding range between 7–8.5, depending on GM1 density [18,19] and GM1 to CT molar ratios [22,23].
The effect of pH on sensor response towards a CT concentration of 100 nM is provided in Figure 3. The maximum response was obtained at pH 7.0, whereas at higher values response decreased dramatically. At lower pH values, CT binding to GM1 is inhibited [19]; the weak response obtained at pH is probably due to CT aggregation at the film surface [24]. Mixed lipid bilayer/GM have shown that the polar GM~ head group has a maximal extension of 15 è from the center of the phosphocholine (PC) group at the membrane surface and that the carboxylate group of the sialic acid moiety is located 10 Å from the PC. The binding of CT to GM1 results in a substantial reorganization of the lipid membrane structure [17].
ConclusionThe preparation of a novel minisensor, based on a polymer stabilized lipid film and an incorporated natural receptor packed on graphene nanosheets. The sensor exhibits long shelf life and adequate operational stability for environmental analysis, whereas it is constructed in a three-step, amenable to commercialization, routine: (i) preparation of graphene electrode, (ii) lipid film UV polymerization and receptor incorporation, and (iii) encapsulation of the lipid film onto the graphene electrode.
The sensor has been successfully validated for cholera toxin detection in water; the results indicate that the GM1-cholera toxin interactions employed herein might serve as a suitable model for developing detection schemes for other receptor-mediated toxin uptake, such as E.coli, Clostridium, Shigella or Pertussis toxins. GM1 receptor can also serve for E.coli toxin detection, whereas polysialic gangliosides might be more appropriate for other toxins [18,25,26].
The method offers fast response times (~5 min) and nanomolar detection levels without significant interference from other water constituents. The limit of detection in the proposed method is 1.0 nM which is comparable or lower to most biosensor systems reported in literature, e.g., the piezoelectric membrane sensor has a detection limit of 2.5 nM and similar response times [27], whereas immunosensing can provide nanomolar detection within 5–8 min at very complex and tedious assay platforms [20,21,28]. The color detection scheme proposed in [29] exhibits a detection limit of 54 nM and a response time of 10 minutes. The SPR CT biosensor reported in [30] exhibits a detection limit of 1 ×10-14 M at optimized conditions, although the time of response is 15 min; similarly, the fluorescence quenching platform proposed in [31] provides a 10 pM detection limit at a response time of 30 min.
A detection limit of 0.06 micromolar has been previously reported for a similar lipid membrane platform [16], although bioelectrode regeneration or real water applicability has not been established. Limits of detection of as low as 45.6 ng/ mL were recently reported in the literature [32]. However, the thin lipid biosensor platforms proposed herein can be easily engineered into portable field detectors for onsite alarm monitoring. The CT detection technique developed offers certain advantages over the commonly preferred immunoassay approaches: (a) the incorporation of the receptor in the lipid membrane platform is performed through a simple polymerization step avoiding any activity loss; (b) the receptor binds rapidly to CT with strong affinity that allows rapid detection; (c) the sensor can be easily and reliably regenerated in a flow system without receptor leakage or memory effects. In effect, the proposed scheme can be easily packed into a portable field detector for rapid water quality preliminary assessment for lifethreatening contaminants and bioterrorism attacks.
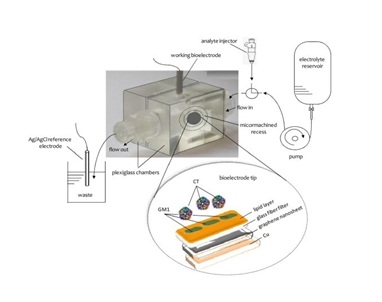
Figure 1: Schematic of the experimental set-up for the detection of cholera toxin [From Reference 16 with permission]
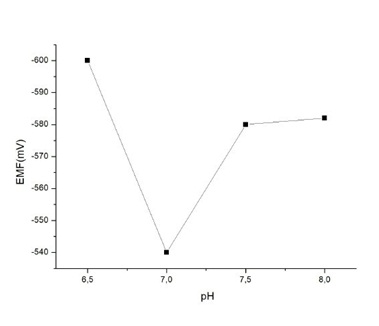
Figure 2: The response of the sensor to 1x10-7 M of cholera toxin over the pH range of 6.5-8.0 [From Reference 16 with permission].
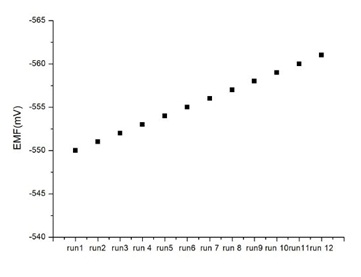
Figure 3: Sensor response to repetitive injections of 7× 10¢8 M CT (pH=7.0); after each response (recorded at 5-min stop flow mode), the sensor was regenerated at 2.0 ml/min flow rate for 6 min before the next CT injection [From Reference 16 with permission]
Chat with us on WhatsApp