
Citation: Ari MM, et al. Gross Responses, Haematological Indices and Serum Biochemistry of Growing Cockerels Fed Diets Containing Graded Levels of Rumen Liquor Fermented Sugarcane Scrappings as Replacement for Maize. J Agri Res 2016, 1(1): 000103.
*Corresponding author: Maikano M Ari, Department of Animal Science, Nasarawa State University Keffi, Nigeria, Email: mari@une.edu.au
This study investigated the gross response, Haematological indices and serum biochemistry of growing cockerels fed diets containing 0, 5, 10 and 15 % of rumen liquor fermented sugarcane scrappings (RFSCS) as replacement of maize in diets of growing Ammo cockerels. At the end of 35 d feeding trial, there was no significant (P>0.05) differences in final weight (FW), weight gain (WG), feed conversion ratio (FCR), survival percentage (%), mean corpuscular volume (MCV) and protein while feed intake (FI), packed cell volume (PCV), haemoglobin (Hb) concentration, mean corpuscular haemoglobin concentration (MCHC), red blood cells (RBC) count, white blood cells (WBC) count, cholesterol, urea, uric acid, glucose, albumin and globulin were affected (P<0.05) by feed treatment. These results showed that RFSCS can replace up to 15% of maize in the diets of growing cockerels without any deleterious effects, 10% replacement of maize with RFSCS is recommended for optimum performance.
Keywords: Cockerels; Performance; Rumen Liquor; Sugarcane Scrapings; Serum
The contribution of livestock sector in the achievement of optimum protein supply, economic returns and sustainable environment in most developing countries will require a continuous search for alternative feed sources that are environmentally friendly, cost effective and in less demand as direct food resource for humans. This is even more important in the production of chickens in spite of their reported potentials of bridging the protein – supply gap in most developing countries, because feeds constitute 70% of production cost [1].
The largest dietary requirements for poultry are energy and protein [2]. These are predominantly supplied by maize grain and soybean meal (SBM) in poultry diets, respectively. The high cost of these feed ingredients resulting from diverse usage in human diets as well as industrial applications makes it necessary to search for alternative replacements that are cost effective. Agroindustrial waste products such as sugarcane scrapings are readily used as non-conventional feeds resource in replacing energy sources such as maize in monogastric diets [2-4]. Sugar cane scrapings are waste produced from scraping the rind of the sugar cane stem with a sharp knife in order to provide easier access to the underlying, soft parenchyma tissue, when the cane is being processed for chewing to extract the cane juice. This scrapings and peels are mostly heaped and sometimes burnt, or left thereby constituting an environmental pollution problem. High energy and crude fiber but low crude protein contents was reported for sugarcane scraping [5,6]. In order to enhance the utilization of agro-waste products in poultry and monogastric diets, the high fiber contents must be broken down and its nutrient composition improved through processing. Various processes have been reported in achieving nutritional improvement, reduction in fiber content of alternative feedstuffs, and these include fermentation, use of exogenous enzymes, mechanical and chemical treatments among others [5,7]. Both in vivo and in vitro fermentation of sugarcane waste and other agro – industrial waste using rumen liquor have been reported [2,8-10] for ruminants and monogastric animals. This fermentation process has been found to have immune stimulating effects on chickens [11].
Even though cockerels plays important role in community poultry upgrade programmes and are considered as good sources of protein meat, commanding high demand because of their meat flavour and toughness [12,13], their feed cost covering twenty (20) weeks poses great challenge to farmers. The use of fermentation using rumen filtrate will provide the opportunity for improving the nutritive values and utilization efficiency of sugar cane scrapings as a substitute to maize in poultry feeds. The basic assumption of this study was that cockerels’ utilization of rumen liquor fermented sugar cane scrapings (RFSCS) will differ with the levels of inclusion. The objective of this study is therefore to evaluate the effect of different replacement levels of maize with RFSCS on the gross performance, haematology and serum biochemistry of cockerels.
Materials and MethodsStudy Area
The experiment was carried out at the Poultry Unit of the Teaching and Research Farm, Faculty of Agriculture, Nasarawa State University Keffi, Shabu-Lafia campus, Nasarawa State located along Latitude 08035’N and Longitude 08033’E in the Guinea savanna zone of North Central Nigeria.
Agro Waste Products collection and processingSugarcane scrapings: Sugarcane scrapings were collected from the local sugarcane marketers who clean up sugar cane sticks for direct chewing by humans in Lafia municipality of Nasarawa State, Nigeria. The scrapings were obtained by scraping the rind of the sugarcane with a sharp knife. The collected scrapings were cleansed and sun dried on a polythene covered concrete slabs for 7d and stored.
Rumen Liquor: The bovine rumen fluid was collected as slaughter waste generated from slaughtering of White Fulani Cows at Lafia Municipal abattoir. The rumen liquor obtained from the slaughtered animals were mixed, homogenized and filtered through 100-mm nylon net. The solid materials were discarded while the fluid (liquor) part of the content was transferred into insulated flasks and stored. The stored rumen liquor was used as the source of inoculums.
Inoculation of Sugarcane scrapping with rumen liquor: The stored rumen liquor was used to inoculate the sun dried and tampered sugarcane scrapings by spraying 6L of rumen liquor per 30 Kg of sugarcane scrapings. The sprayed sugarcane scrapping was thoroughly mixed and transferred into airtight plastic container to initiate fermentation process that lasted for72 h. Temperature, pH and texture of product were recorded at 24 h intervals. The fermented samples were sundried and analysed for nutrient composition according to [14] methods.
Management of experimental birdsA total of two hundred and eighty- eight (288) – twelve (12) weeks old day - old Ammo cockerels raised on commercial chick mash (1- 30 d) and growers’ mash (30 – 84 d) were used for the experiment. The growing cockerels were individually weighed and randomly assigned in a Completely Randomized design to Four (4) dietary treatments of six (6) replicates each. Each replicate had twelve (12) birds. The birds were reared on deep litter in an open sided well-ventilated poultry house. Routine management practices and routine administration of vaccines against Infectious Bursal Disease and New Castle Disease were undertaken and the birds were fed ad libitum and had access to clean water.
Experimental diets: During the 35 d feeding trial, four (4) experimental diets were formulated to provide approximately 2800 Kcal/ME and 14% CP using Feedwin soft least cost soft ware (table 1).
Data Collection: Data collected during and the conclusion of the experiment include: feed intake, weight gain, feed conversion ratio, and survival percentages as measures of gross response. At the end of the feeding 35 d trial, blood samples were collected from four (4) birds per replicate using 5mls sterile disposable syringes and needles under the jugular veins in two (2) containers each with and without EDTA, blood samples collected were analyzed for haematological indices and serum biochemistry.
Analytical ProceduresProximate analysis: Chemical compositions of each of the experimental diets were determined following standard methods [14].
pH and Temperature: pH and temperature values for fermentation of sugarcane scrapings using rumen liquor were determined after every 24 h using Hm Digital Hand Held TDS – 3 tester.
Blood analysis: Blood samples were analysed for haematological parameters including haemoglobin (Hb), packed cell volume (PCV), white blood cell (WBC) count and Red blood cell (RBC) count. The cell counts were carried out by the use of haemocytometer while Hb, PCV and serum chemistry indices were determined using methods adopted by [15] and the methods described by [16,17].
Statistical analysis: Data collected were subjected to one-way analysis of variance (ANOVA) using SPSS 20, Means were separated where there were significant differences using Duncan’s Multiple Range Test [18].
Results and DiscussionThe proximate composition of sugar cane scrapings with and without fermentation with rumen liquor is presented in (table 2).
The values of DM, NFE increased with fermentation while CP, EE, CF and total ash were reduced with fermentation activity. The observed variations in the composition of between fermented and unfermented sugarcane scrapings is accounted for by the effects of fermentation [7,19] through the used up and reconstitution of nutrient resource by fermenting microbes especially during the process of crude fiber degradation [20]. There were variations in fermentation temperature and slightly pH with increased length of fermentation time (24 h to 72 h) which was similarly observed with in vivo fermentation of other agro waste products [10,21]. The composition of the experimental diets (Table 3).
The gross responses of the experimental birds are presented in (Table 4).
The experimental birds did not differ (P<0.05) significantly in Final weight (FW), Weight Gain (WG), Feed Conversion Rate (FCR) and Survival percentage while they differ (P<0.05) in feed intake (FI). Complete maize diet however had higher FI, FW and WG compared to other treatments while had the lowest values for the same parameters. These results support the usability of both sugar cane scrapings and the process of its nutritional improvement earlier reported [5,20,22].
The reduction in feed intake for 15% group may be as result of metabolites produced as bye products of fermentation process, thus with increased replacement of maize with RFSCS, these metabolites will increase in the feed and invariably reduce intake by birds. The high survival rate recorded for all groups confirms the report of [15,23] on the hardiness of cockerels and that of [11] who reported immune stimulation ability of sugar cane extracts in diets.
Results of haematological parameters and serum biochemistry are presented in (Table 5).
Significant (P< 0.05) differences were observed among treatments for packed cell volume (PCV), haemoglobin (Hb) concentration, mean corpuscular haemoglobin concentration (MCHC), red blood cells (RBC) count, and white blood cells (WBC) count, while mean corpuscular volume did not differ (P<0.05) significantly between dietary treatments. Even though the observed pattern of variations does not follow any particular trend in the rate of substitution of maize with RFSCS in the diet, PCV and MVC values decreased with increase in replacement of maize. The Haematological values obtained in this study were all within the reported normal ranges for chickens [15,24,25]. This suggest that the replacement of maize as energy source with up to 15% RFSCS was not deleterious to blood formation activities and its indicative of the nutritional potentials of RFSCS in the diets of cockerels [26,27]. The reduction in the values of haematological indices like PCV, Hb, MCV obtained in this study are supported by the findings of [28] who observed similar trend in broilers when fed rumen liquor fermented feed product which may be associated with poor intake resulting from the presence of metabolites in these diets [29].
The serum biochemical indices of the experimental birds showed significant (P< 0.05) effects on the following indices; cholesterol, urea, uric acid, glucose, albumin and globulin while values protein were not affected (P<0.05) by feed treatment. These variations do not follow any pattern, however values for uric acid increased with increased utilization of RFSCS in the diets.
Cholesterol values were highest and lowest (P< 0.05) in the 10 and 5% replacement of maize, these values are within range reported for cockerels [15] and that of [30]. The total protein values obtained ranged from (3.05 – 3.65 g/dl) with 0% and 15% replacement diets having the lowest and highest values respectively the total protein of the experimental diets compared with that (3.60 - 4.0g/dl) reported for chickens [15,31]. Uric acid values was particularly high with increased replacement levels of RFSCS in the diet (116.50 -197.50 mmol/l) for 0 and 15% inclusion levels. This finding is at variance with the report of [32] who reported a significant decrease of uric acid concentration in fasted cockerels, bearing in mind the low feed intake recorded for 15% replacement group. The values are however lower than the one reported. Glucose similarly increased with increased replacement with rumen liquor fermented sugar cane scrapings; this was supported by (El-Abasy et al., 2002) [11] who observed increased role of sugars in blood and immune response of cockerels. The albumin levels obtained in this study (1.65 – 2.15g/dl) for (15 and 0% respectively) compared favorably to the normal range (1.70 – 2.0g/dl) reported [15,33] for chickens while the globulin levels was highest in 10% replacement group (1.73 g/dl) and the values obtained in this study were within similar range (1.35 – 1.75g/dl) observed by [15,34] but were however, lower than the normal range (2.0 – 2.90g/dl) reported by the same workers.
ConclusionThese results obtained from this study showed that RFSCS can serve as up to 15% energy replacement of maize in the diets of growing cockerels without any deleterious effects gross performance and blood parameters of cockerels. For optimum performance, 10% replacement of maize with rumen liquor fermented sugar cane scrapings is recommended.
AcknowledgementThe authors gratefully acknowledge the FAC Research Grant, Mr. N.A. Tsaku and Mrs. Ramatu Aliyu (Laboratory Technologists), as well as staff of the Live stock Research and Demonstration Farm of the Faculty of Agriculture, Nasarawa State University, Keffi for their technical assistance during the course of this research work.
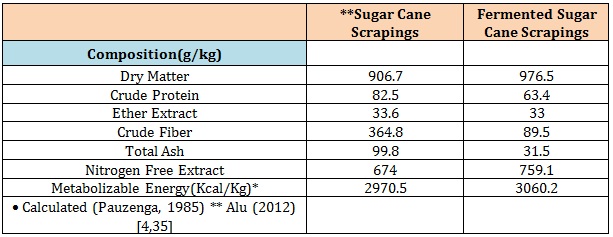
Table1: Proximate composition of sugarcane scrapings.
The Four (4) dietary treatments are D1, D2, D3 and D4 representing 0, 5, 10 and 15 % replacement of maize with RFSCS.
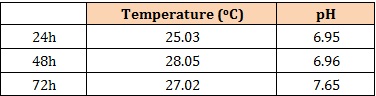
Table 2: Effects of fermentation time on temperature and pH.
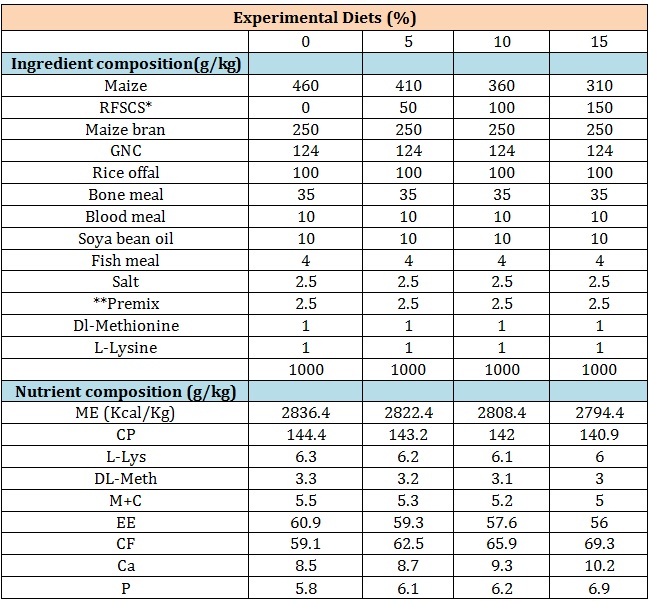
Table 3: Composition of experimental diets.
*Inclusion levels of Rumen Liqour Fermented Sugar cane Scrapings
**Premix contains Vitamin A (8,000,000 I.U);Vitamin D3 (2,000,000 I.U); Vitamin E (5,000mg); Niacin (15,000mg); Vitamin B1 (1,500mg); Vitamin B2 (8,000mg); Vitamin B6 (1,500mg); Vitamin B12 (10mg); Vitamin K3 (2,000mg); Calpan (5,000mg); Biotin (20mg); Folic acid (500mg); Antioxidant (125,000mg); Choline chloride (200,000mg); Cobalt (200mg); Copper (5,000mg); Iodine (1,200mg); Iron (40,000mg); Manganese (80,000mg); Selenium (200mg); Zinc (60,000mg).
showed that the formulated experimental feeds are within the recommended nutritional requirements values for growing cockerels
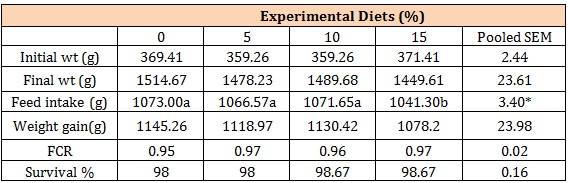
Table 4: Gross Response of cockerels fed experimental diets.
*Inclusion levels of Rumen Liqour Fermented Sugar cane Scrapings
abc Means with different alphabets in the same row were significantly (P<0.05) different
SEM Standard error mean
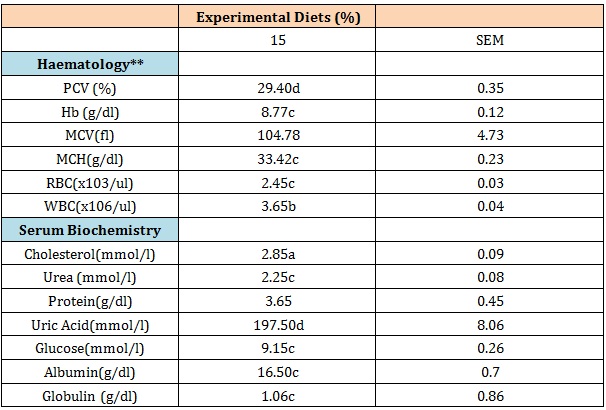
Table 5: Haematological and serum biochemistry of experimental cockerels
*Inclusion levels of Rumen Liqour Fermented Sugar cane Scrapings
Abc Means with different alphabets in the same row were significantly (P<0.05) different
SEM Standard error mean
**PCV: Packed Cell Volume; Hb: Haemoglobin; MCV: Mean Corpuscular Volume; MCHC; Mean Corpuscular Haemoglobin Concentration; MCH: Mean Corpuscular Haemoglobin; RBC: Red Blood Cell; WBC: White Blood Cell.
Chat with us on WhatsApp