
Citation: Khairi M, et al. Composting Enhanced Manganese-Induced Sweet Potatoes Production in Sandy Soil. J Agri Res 2016, 1(2): 000107.
*Corresponding author: Md Sarwar Jahan, Faculty of Bioresources and Food Industry, Universiti Sultan Zainul Abidin, (UNISZA), Malaysia, Tel: +6-09-699-3453; Email:sarwarjahan@unisza.edu.my
Composting retains different concentrations of nutrients, which are important in increasing health of sandy soil for sustainable production of crops. To examine the effects of compost on manganese (Mn)-induced sweet potato production, different plants parameters of sweet potato were measured. A foliar application of diverse concentrations of Mn (0.0, 0.2, 1.5 and 3.0 ppm) with or without composting was justified for the growth and development of sweet potato plants. Compost enhanced leaf area, chlorophyll (Chl) content, relative water content (RWC)of leaves, moisture content of the tuber, photosynthesis rate, tuber numbers, tuber weight, the content of ash, crude fiber, protein of tuber regardless of Mn application. Manganese concentration at 1.5ppm showed improved results of above parameters compared to the control and other concentrations of Mn. These results indicate that composting might increase the health of sandy soil, which endure the production of sweet potato in low fertile soil.
Keywords: Chlorophyll contents; Leaf area; Relative water content; Photosynthesis; Crude fiber; Protein; Sweet potato
Sweet potato (Ipomoea batatas L.) is a leading crop in the world that provides CHO for human health and relatively easy to grow and productive under the low fertile soil [1]. Compost includes different plant nutrients [1] that improve soil fertility and sustain crop yields [2]. Ahmed et al. [3] suggest that compost as a soil amendment increases crop production. Organic matter manages low fertile soil for sustainable agriculture production [4] and improving soil quality [5]. Organic matter increases water retention capacity in sandy soil and defends plants in contrast to the drought stress by promoting beneficial microbes [6]. In addition, compost decreases soil-borne diseases and pollution and reduces runoff and leaching of essential nutrients [4].
Manganese (Mn) is a plant micronutrient functions on plant metabolic factors such as respiration, amino acid synthesis, photosynthesis and hormone activation [7]. Excess of Mn concentration affects enzyme activity, metabolic functions of plants and creates oxidative stress [8]. Mn is a co-factor for most antioxidant enzymes in plants [9]. Mn affects growth of plants, Chl content, antioxidant activity and elongation of root and shoot of plant [10], photosynthesis production [11], superoxide dismutase and catalase activity [12] and reactive oxygen species [13,14].
The earlier report showed that compost improved the soil health of the sandy soil [15] to sustain crop production [2]. To date; no research was conducted on the effects of composting on Mn-induced sweet potato production. Therefore, this study focused to justify physiological parameters of corn plants in relation to the Mn-induced sweet corn production. The objective of this research was to determine the effect compost on Mninduced growth and production of the sweet potato plants. We showed that compost increased Mn-induced tuber production through enhancing physiological functions of the plants.
Materials and Methods Materials and experimental designThe sweet potato (Ipomeabatatas L.) plant is used for this experiment is belonging to the family of Convolvulaceae. Four manganese concentrations (0, 0.2, 1.5 and 3.0 ppm) were applied to the plants grown under the conditions of with or without compost. Treatments are arranged as per completely randomize design with five replicates. Care of plants [16,17] and different cultural practices were followed according to the previous studies [18,19].
Land preparation and fertilizer application
Compost was mixed with soil properly and stabilizes for two weeks before planting of vines, which were used as planting material of sweet potato [1]. The prepared land was constructed into the bed where vines were planted in the distance of 30 cm between two veins. The inorganic fertilizers such as nitrogen (100 kg) phosphorous (90 kg) and potassium (200 kg) per ha were applied according to the guideline provided by the Department of Agriculture, Malaysia. During tuber development, a 300 kg of NPK blue (12:12:17 + TE) was applied to boost tuber production.
Determine of leaf areaA LP-80 leaf area meter is used to measure the leaf area of plants according to the manual.
Determine of relative water content of leaves and moisture contents of tuberRelative water content of leaves was measured as per previous studies [20,21]. We detached leaves the plants then immediately measured fresh weight (FW). Then leaves were incubated in distilled water for 24 hours then dabbed dry using soft tissue paper to obtain the turgid weight (TW). The dry weight (DW) of leaves was measured after oven dried at 65°C for 24 hours. The formula of RWC (%) = [(FW-DW) / (TW-DW)] x 100 was used to calculate relative water content (RWC) of leaves sweet potato. The moisture content of tuber was analysed as per the previous method [22]. The following formula was used to calculate the moisture contents of tuber.
Percentage Moisture = {(W2 –W3) x 100} x (W2 –W1)
Where W1 = weight of empty crucible (g); W2 = weight of crucible + sample prior to drying (g) and W3 = weight of crucible + sample after drying (g).
Determine of chlorophyll content and net photosynthesis rate of leavesA portable SPAD chlorophyll meter was used to determine chlorophyll (Chl) content of leaves as per previous study [23]. Measurements were taken using young, fully expanded leaves with five replicates.Net photosynthesis rate (pn; µmol/m2/s)was measured using a portable gas exchange fluorescence system (CI-340 Handheld Photosynthesis System) according to the manual and previous studies [24,25]. Measurements were taken between 11:00 am and 1:00 pm to avoid the wetness condition on the leaf. Five replicates were maintained for each treatment.
Determination of Proximate AnalysisThe sweet potatoes were cleaned and a 100 g of sweet potatoes was cut into small pieces and placed into the drying oven at 65°C for 72 hours. After that, the sample was ground using a commercial blender. The proximate analysis comprised the following assay, ash contents, crude protein, fiber which were assess using the standard methods of Association of Official Analytical Chemists [26].
Determination of Yield ParametersYields as tuber weight (wet and dry condition) were estimated using a weighting balance.
Statistical analysisData were analysed by the ANOVA procedure and differences of mean among treatments were analysed by the SPSS software. Differences at P value <0.05 was considered significant.
Results Effects of compost on Mn-induced leaf area of sweet potato plantsData of leaf area of sweet potatoes was presented in Figure 1.
Mn application did not affect leaf area compared to the control condition (Figure 1; closed bars). In contrast, compost increased leaf area of sweet potato plants but showed a similar effect in all treatments. Results confirmed that application of compost significantly increased leaf area (Figure 1; open bars) regardless of Mn application.
Effects of composting on Mn-induced chlorophyll contents of leavesThe light antenna in photosystem II affects Chl content 22which consistent with the net photosynthesis rate in leaves of plants [25,27]. Mn application significantly increased Chl content in leaves of sweet potato plants than control (Figure 2; triangle).
This result was consistent with the previous study [28]. Compost-treated plants accumulated significantly higher Chl content than compost-untreated plants. In addition, 0.2 ppm of Mn increased Chl content other Mn concentrations (Figure 2) but higher Mn concentration showed a similar result. In addition, under 1.5 and 3 ppm Mn conditions, compost has no effect on Chl content. These results indicate that composting increased Chl content in leaves that might modulate the function of a light antenna in photosystem II to affect photosynthesis activity in plants.
Effects of composting on Mn-induced relative water content in leaves and moisture content in tuberFigure 3 shows RWC of leaves and moisture content of tuber of sweet potato plants treated with compost and Mn.
Figure 3a shows that Mn has no effect on RWC of leaves and showed a similar to that of the control treatment. Furthermore, compost increased RWC of leaves of sweet potato plants regardless of Mn treatments (Figure 3a; open bars). In case of the moisture content of tuber, Mn and compost showed consistent results to that of RWC of leaves of sweet potato plants (Figure 3b). In comparison data between Mn and compost application, compost significantly increased moisture content of tuber than that of compost-untreated plants. These results suggest that composting in sandy soil might increase soil health, such as water holding capacity, reduce soil temperature, which might increase RWC of leaves and moisture content of tuber of sweet potato plants.
Effects of compost application on Mn-induced photosynthesis rateFigure 4 shows net photosynthesis rate of leaves of sweet potato plants treated with composting and Mn. The treatment of Mn at 0.2 ppm increased net Pn rate of leaves compared to the control and higher Mn concentration (3 ppm of Mn; Figure 4; closed bars).
This result suggests that Pn rate is dose-dependent of Mn content that is increasing Pn rate as favour of increasing Mn concentration but decreased when Mn concentration is high. Moreover, compost significantly improved Pn rate of leaves regardless of Mn treatments (Figure 4a; open bars) but showed a similar trend to that of the Mn-treated Pn rate of plants. This result suggests that compost application in sandy soil improved soil health, which might increase Pn rate of leaves of sweet potato plants indicating improved cell functioning.
Effects of compost application on Mn-induced number and weight of tuberWhether composting affects Mn-induced sweet potato production we measured numbers of tuber and the weight of tuber after harvested of the tubers. Figure 5a shows that 1.5 concentration of Mn application affected numbers of tuber compared to the control.
In contrast, compost application significantly induced numbers of tuber than Mn-untreated and -treated plants (Figure 5a; under 0 ppm of Mn). But compost application did not affect numbers of tuber when data were compared between Mn-treated and Mn-untreated plants. These results indicate that compost-induced numbers of tuber production was not affected by the different concentrations of Mn (Figure 5a). Weight of tuber was consistent with numbers of tuber (Figure 5b). This result suggests that compost induced Mn-induced corn production in low fertile soil. Effects of compost on Mn-induced ash, crude fiber, and crude protein of tuber
Figure 6 shows the effects of compost application on ash, crude fiber and protein content in Mn-induced tuber of sweet potato plants.
Mn treatment (0.2 ppm) significantly increased ash content of tuber than control and other Mn treatments (Figure 6a; closed bars). Similarly, compost increased ash content of tuber regardless of Mn application (Figure 6a; open bars). Crude fibre (Figure 6b) and protein content (Figure 6c) of the tuber were similar to the ash content of tuber. This result indicates that compost application increased Mn-induced ash, crude fiber, and protein content of tuber of sweet potato plants.
DiscussionSandy soil has a gritty texture that lower water and nutrients holding capacity to create detrimental effect for most of field crops. Compost improves sandy soils structure, which improve nutrients availability from where plants absorb nutrients. Finished compost contains different amounts of plant nutrients, which improve plant growth [29] and soil health [24]. In this study, it showed that compost application increased the leaf area of plants regardless of Mn treatment (Figure 1). But different concentrations of Mn did not affect leaf area, which is consistent with previous results [30]. Therefore, it is important that the health of sandy soil to be improved for sustainable crop production by the application of compost. In this study, application of compost increased Mn-induced leaf area (Figure 1) indicating the improvement of soil health to enhance plant growth. This result clues us to explain the effects of compost on Mninduced physiological consent and production of sweet potato plants.
Chlorophyll content and Chl fluorescence are associated with the GSH content and light antenna function in plants [31,32,23]. These results indicate that light related parameters control the growth of plants, which might, in turn, be regulated by the Chl function in leaves. Dry soil condition condensed Chl content in leaves to controls crop productivity through CO2 assimilation [33] while sandy structure of soil reduces phyto availability of nutrients in soil [2]. Above results showed reliable with this study that plants accumulated lower Chl content under control and sandy soil condition (Figure 2). These results indicate that water stress that is induced by sandy soil might affect Chl-related plant growth and development [2]. In addition, Chl content is correlated with glutathione (GSH) content in plants [34-36]. Increment of Chl content under composting condition indicates of higher GSH accumulation which might provide defense mechanism to the plants under low fertile soil for sustainable growth.
Different conditions such as less water [21], salinity [31], less nutrients (Mn and Cu) and sandy soil [35,28,25] affects RWC of leaves. We demonstrated in this study that application of compost in sandy soil enhanced RWC in leaves of sweet potato (Figure 3a). This result suggests the application of the compost might improve sandy soil health, which coordinates with the higher RWC in leaves. This result suggests the well plant metabolic reaction that higher photosynthesis rate and proper cells functioning in plants [35]. Low water in leaf causes substantial oscillations of light energy transformation in photosystem [37] which might important for plant physiological parameters that are enhanced by the application of Mn and compost.
The photosynthesis rate of sweet potato plants showed dose dependency of Mn (Figure 4) which affect compostinduced Pn rate in plants. This result is consistent with photosynthesis activity in leaves [38]. Therefore, it is imaginable that compost-induced Pn rate of leaves of sweet potato (Figure 4) might enhance other physiological functions such as stomatal conductance and the transpiration rate [39] and cell membrane integrity [40]. Compost application in soil increases microbial activity, nitrogen concentration that leads to increase grain yield [41]. Compost might induce soil health to sustain the production of potato plants (Figure 5a), where Mn may play a role in aggregate plant functions for sustainable production.
In conclusion, sandy soil is detrimental to the growth of field crop and ultimately decreases production due to very low soil health. Therefore, it is possible that compost increased Mn-induced physiological functions of sweet potato plants to improve the soil health sustainable production of tuber.
AcknowledgementThis work was supported by grants the faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin.
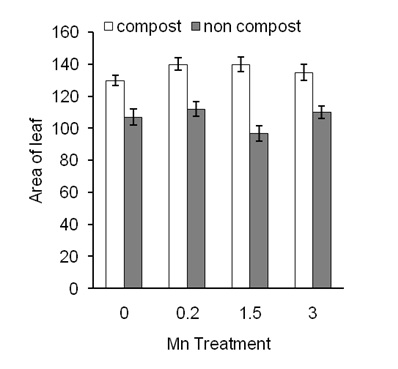
Figure 1: Effects of different concentration of Mn on area of leaf in the presence of compost (open bars) and absence of compost (closed bars) of sweet potato plants. Error bars indicate standard deviation with 5 replicates.
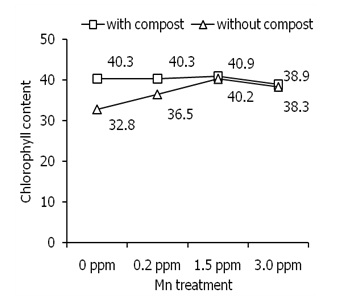
Figure 2: Effects of different concentration of Mn on chlorophyll content of leaves of sweet potato in the presence of compost (open square) and absence of compost (open triangle). Error bars indicate standard deviation with 5 replicates.
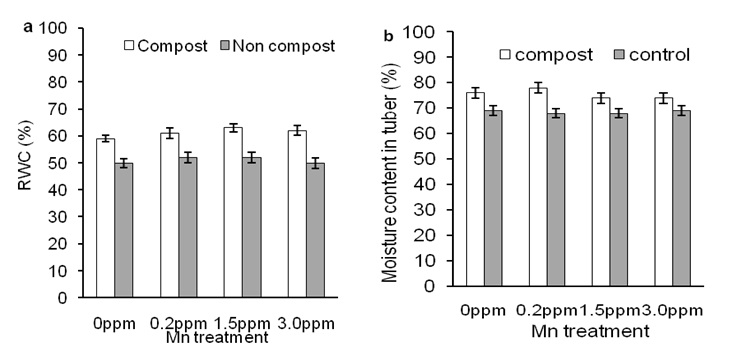
Figure 3: Effects of different concentration of Mn on relative water content in leaves (a) and moisture content of tuber (b) of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
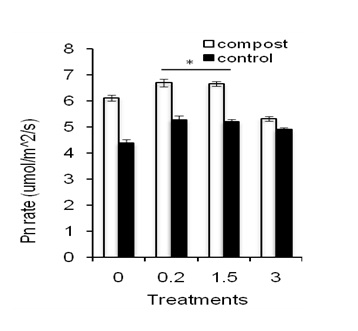
Figure 4: Effects of different concentration of Mn on photosynthesis rate in leaves of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
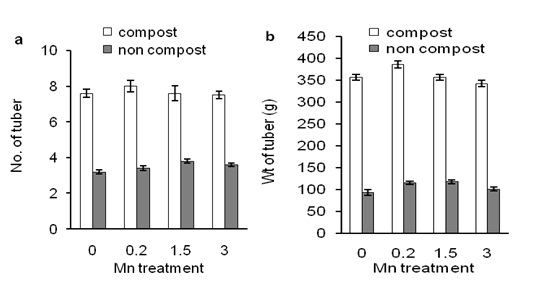
Figure 5: Effects of different concentration of Mn on numbers of tuber (a) and weight of tuber (b) of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
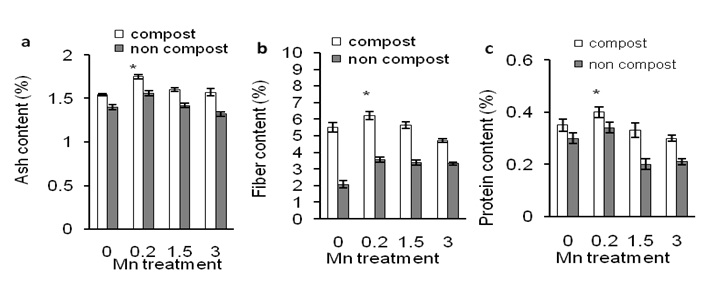
Figure 6: Effects of different concentration of Mn on ash content (a), fiber content (b) and protein content (c) of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
Chat with us on WhatsApp