
Citation: Khairi M, et al. Compost and Zinc Application Enhanced Production of Sweet Potatoes in Sandy Soil. J Agri Res 2016, 1(2): 000108.
*Corresponding author: Md Sarwar Jahan, Faculty of Bioresources and Food Industry, Universiti Sultan Zainul Abidin, (UNISZA), Malaysia, Tel: +6-09-699-3453; Email: sarwarjahan@unisza.edu.my
Composting releases nutrients in the less fertile soil to increase soil health for sustainable production of a crop. To investigate the effects of compost-and zinc (Zn)- induced growth and development of sweet potato, we measured different parameters of sweet potato. Different concentrations of Zn (0.0, 0.2, 1.5 and 3.0 ppm) were applied as a foliar spray to the plants with or without composting. Compost increased leaf area, chlorophyll content, relative water content in leaves, moisture content in the tuber, photosynthesis rate, numbers of tuber, the weight of tuber, ash, crude fiber and crude protein in tuber of sweet potato plants regardless of Zn application. Zinc concentration at 0.2ppm showed better results in the above parameters compared to the control and higher concentration of Zn. These results confirmed that composting might increase sandy soil health to sustain the production of sweet potato in sandy soil.
Keywords: Chlorophyll contents; Leaf area; Relative Water content; Photosynthesis, Crude fiber; Crude protein
Sweet potato (Ipomoea batatas L.) is one of the leading crops in the world for human food, which became a staple food in Malaysia. It is relatively easy to grow, free of pests and diseases and is productive under the less fertile soil [1]. Zn works as a cofactor for about 300 enzymes and proteins involved in cell division, nucleic acid metabolism and protein synthesis. Zinc increases the chlorophyll content to induce the growth of cabbage [2]. Zinc enhanced production in glutathione-treated corn plants through improvement in physiological functions [3].
Composting encompasses different essential nutrients [1] to maintain soil fertility, improves and maintains crop yields and prevents sweet potato tubers from rotting [4]. The previous finding suggests that compost could be used as a soil amendment to increase crop production on a sustainable basis. Management of less fertile soil by the application of organic matter is a key for sustainable agriculture production [5] and increasing soil organic matter for improving soil quality [6]. Organic matter helps to retain water in the sandy soil and protects plants against drought stress by promoting biological activity of the soil due to increasing beneficial microbes [7]. Moreover, compost reduces soil-borne diseases and pollution by reducing nutrient runoff and leaching [5].
Zinc is an important micronutrient necessary for corn production. It is involved in many physiological processes in plants. Deficiency and excessive application of Zn affect growth and development of plant [8], leaf chlorosis and reduced size of leaves, and sterility of spikelet [9]. There is a relationship between glutathione and Zn that works as an excellent activator of the enzyme to initiate the formation of phytochelatin that induces Zn phyto availability in plants [10]. Zinc stabilizes and protects bio membranes against oxidative stress [11], modulates plants’ antioxidant systems to reduce free radicals damage effects [12].
However, few studies have evaluated effects of compost and Zn fertilizer management on physiology and yield of sweet potato plants in sandy soil. The previous report stated that the application of compost improved crop yield in an amended sandy soil with compost by improving the soil properties [13]. Therefore, the objective of this research was to understand the effect of compost and zinc increased growth and production of the sweet potato plants.
Materials and MethodsThe sweet potato, known as Ipomeabatatas L. was chosen for this experiment. The plant belongs to family Convolvulaceae. There were four zinc concentrations (0, 0.2, 1.5 and 3.0 ppm) in combination with or without composting according to the completely randomized design with five replicates. Care of plants [14,15] and different cultural practices were followed according to the previous studies [16,17].
The inorganic fertilizers, 100 kg of nitrogen, 90 kg of phosphorous and 200 kg of potassium, were applied according to the guideline provided by the Department of Agriculture, Malaysia. At the time of the development of tuber, NPK blue (12:12:17 + TE) was applied at 300 kg per ha. Compost was applied before planting and mixed with soil properly and stabilize for two weeks before vine planting. The tropics sweet potato is produced from cutting of existing vines to form planting material [1]. The vine was made up of nodes, bumps on the vine where leaves develop. Soil bed was prepared and vines were planted at a distance of 30 cm.
Leaf area was measured by a LP-80 leaf area meter. Relative water content was estimated following previous studies [18,19]. The fresh weight (FW) of leaves was measured immediately after leaves were detached from the plants followed by incubation in distilled water for 24 hours. Then, the turgid weight (TW) was recorded after leaves were dried using soft tissue paper. Leaves were then oven dried at 65°C for 24 hours after that dry weight (DW) was recorded. The relative water content (RWC) of leaves was analyzed by the following formula of RWC (%) = [(FW-DW) / (TW-DW)] x 100.
The moisture content was measured according to the previous method [20]. The fresh weight and dry weight were taken followed by the calculation of the moisture content using the following formula. Percentage Moisture = {(W2-W3) x 100} x (W2-W1)
Where W1 = weight of empty crucible (g); W2 = weight of crucible + sample prior to drying (g) and W3 = weight of crucible + sample after drying (g).
Chlorophyll (Chl) contents in leaves were determined using a portable SPAD chlorophyll meter [19]. Net photosynthesis rate (pn; µmol/m2/s) was measured using a portable gas exchange fluorescence system (CI-340 Handheld Photosynthesis System) according to the manual and previous studies [21,3]. Measurements were taken between 11:00 am and 1:00 pm to avoid the wetness condition on the leaf. The sweet potatoes were cleaned and air-dried before use. A 100 g of potatoes was cut into small pieces and placed into the drying oven at 65°C for 72 hours. After that, the sample was ground using blender. The ash contents, crude protein and fiber content were determined using the standard methods of Association of Official Analytical Chemists [22]. Yield as tuber weight (wet and dry condition) was estimated using a weighting balance. Statistical analysis such as Analysis of Variance (ANOVA) and mean separation were done SPSS software [23].
ResultThe application of compost significantly increased leaf area (Figure 1; under 0 ppm of Zn) compared to noncompost condition (Figure 1 closed bars under 0 ppm of Zn).
Zn application also increased leaf area compared to the control condition (Fig. closed bars). In addition, Zninduced leaf area was higher in compost-treated plants than that of compost-untreated plants. These results show that compost increased leaf area regardless of Zn application. Similar results were observed in the case of the length of a leaf (Figure 1; line graph). Taken together, Zn and compost increased area and length of the leaf. The light antenna in photosystem II affects leaf growth of Arabidopsis [24] that might related to the Chl content and photosynthesis rate in leaves of plants [3,25].
Figure 2 shows that Zn at 0.2 ppm application significantly increased Chl content in leaves of sweet potato plants than other Zn concentrations (Figure 2; closed bars).
This result is consistent with the previous study [3]. In the presence of compost, plants accumulated significantly higher Chl content regardless of Zn application. In addition, 0.2 ppm of Zn showed the better result than other Zn concentrations (Figure 2; open bars). These results indicate that composting increased Chl content in leaves that might modulate the function of a light antenna in photosystem II.
Figure 3 shows RWC in leaves and moisture content in tuber of plants treated with compost and Zn.
Figure 3a shows that 0.2 ppm of Zn showed significantly higher RWC in leaves than other Zn treatment while a higher concentration of Zn (3 ppm) and control treatments showed a similar result. Furthermore, compost application not only increased RWC (Figure 3a; under 0 ppm of Zn) but also increased RWC in leaves of Zn- treated sweet potato (Figure 3a; compared between open and closed bars). In addition compost-treated RWC in leaves was similar and irrespective of Zn application (Figure 3a; open bars). In a case of the moisture content of tuber, both of Zn and compost alone did not affect moisture content (Figure 3b). This result is consistent with RWC content in compost-treated plants (Figure 3a). When data were compared between Zn and compost application, we found that compost significantly increased moisture content in tuber than that of compost-untreated plants. These results suggest that composting in sandy soil might increase soil health, such as water retention capacity, reduce soil temperature, to increase RWC in leaves of sweet potato plants.
Figure 4 shows net photosynthesis rate in leaves of sweet potato plants treated with composting and Zn.
The concentration of Zn at 0.2 ppm significantly increased net Pn rate in leaves compared to the control and higher Zn concentration (3 ppm of Zn; Figure 4; closed bars). This result suggests that Pn rate in plants is Zn- depended where low concentration increased Pn but high concentration decreased Pn rate in leaves of plants. Moreover, compost application significantly increased Pn rate in leaves regardless of Zn treatments (Figure 4a; open bars). These results suggest that composting in sandy soil increases cell functioning in leaves to increase Pn rate in leaves of sweet potato plants. Whether composting and Zn affects the production of sweet potato, a number of tubers and the weight of tuber were measured after harvesting of crop.
Figure 5a shows that Zn application did not affect number of tuber compared to the control. In contrast, compost application significantly increased numbers of tuber in Zn- untreated plants (Figure 5a; under 0 ppm of Zn).
In addition, compost application significantly increased numbers of tuber in 0.2 ppm of Zn-treated plants than other Zn- treated plants. These results indicate that compost-induced numbers of tuber production was differed by different Zn concentrations (Figure 5a). Figure 5a also shows that lower concentration of Zn (0.2 ppm) showed better results than higher concentrations of Zn (1.5 and 3 ppm). Consistently, Zn- induced weight of tuber of plants was consistent with a number of tubers per plants in the condition of composting (Figure 5b). In addition, Zn (0.2 ppm) significantly increased weight of tuber than that of control and higher Zn concentration (3 ppm) but similar to 1.5 ppm of Zn. These results suggest that compost enhanced Zn- induced corn production, and numbers of tuber influenced the weight of tuber of sweet potato plants.
Figure 5 shows the effects of composting on ash, crude fiber and crude protein in Zn- induced tuber of sweet potato plants. Zn treatment at 0.2 ppm concentration significantly increased ash content in tuber compared to that of higher concentration of Zn (3 ppm) and control treatment (Figure 6a; closed bars).
This result indicates that Zn (0.2 ppm) increased ash content in tuber but high concentration decreased the ash content. Composting did not affect ash content in tuber regardless of Zn application (Figure 6a; open bars). Crude fibre in the tuber of Zn- treated plants was similar to the Ash content but composting increased crude fibre regardless of Zn treatments. This result indicates that composting affect crude fiber content in tuber of sweet potato. Crude protein content in tuber (Figure 6c) was similar to the ash content. Taken together, these results suggest that compost has little effect in Zn- induced plants to increase ash, crude fiber, and crude protein in the tuber.
DiscussionSandy soil has a gritty texture and tends to have large particles and water and nutrients holding capacity is very low which is detrimental to survive for many plants. Compost improves the structure of sandy soils that enhance water retention to dissolve nutrients from where plants able to use dissolved nutrients. m Finished compost contains small amounts of essential plant nutrients, which are essential to improve plant growth and development [26]. On the other hand, Zn involved in membrane integrity, enzyme activation, and gene expression [27]. Zinc deficiency causes leaf mottling, interveinal chlorosis, and reduced plant growth [28]. Zn interferes with essential physiological processes but excess Zn cause toxicity on plant and inhibits growth [29] and Zn deficiency induces oxidative stress that affects enzymes activities in carbohydrate metabolism [28]. In this study, we showed that Zn application increased the leaf area compared to the control condition (Figure 1) is consistent with the previous study [30]. This result indicates of increasing photosynthesis rate due to the increasing leaf area. It is important that plants may able to increase photosynthesis products production e.g. sugar. In addition, application of compost increased Zn- induced leaf area (Figure 1) indicates of improving soil health to enhance plant growth. This result leads us to justify the effects of compost on Zn-induced sweet potato production.
Compost and Zn both significantly increased Chl content in leaves of sweet potato plants (Figure 2). This result indicates that Zn application might increase the photosynthesis process [31] which is positively correlated with Chl content [32]. Zinc acts as a component of proteins and as co-factor of enzymes for normal development of pigment biosynthesis [33] that leads to increasing Chl content in leaves in regardless of composting (Figure 2; [34]. Excess of Zn induces chlorosis, chloroplastic damage, membrane rupture and thylakoid disintegration, which affect photosynthesis process [35]. Therefore, Chl content is an important parameter in plant physiological research to estimate the primary productivity and effect of environmental stresses [36].
Zinc is essential for maintaining osmotic potential and water saturation in leaves of plants [2], which relates transpiration streams in plant cell and affect photosynthesis rate [31]. Less water application affects RWC [19] that was consistent with the effects of salinity in rice plants [37] and nutrients (Mn and Cu) on RWC in corn plants [38,39]. In this study, it was demonstrated that application of Zn enhanced RWC in leaves of sweet potato, which was accelerated in the presence of compost (Figure 3a). This result suggests that compost might improve soil health that coordinates higher RWC in leaves. Higher water content in leaves indicates of higher photosynthesis rate and cells functioning well while plant metabolic reaction [40]. In addition, compost confers higher moisture content in tuber correlates higher RWC in leaves under composting condition (Figure 3b).Low leaf water potential causes significant oscillations of light energy translocation in photosystem [41]. These results together confirm that water content in leaf and tuber are important plant physiological parameters that are enhanced by the application of Zn and compost.
Low concentration of Zn in spinach is related to the reduction of photosynthesis, which affects biochemical reactions, transpiration, photosynthesis, chlorophyll biosynthesis and cell membrane integrity [42], electron transport in photosystem II [43]. The photosynthesis in plants was dose dependent of Zn where Pn increased at optimum condition, but excess Zn declined Pn in leaves (Figure 4). This result consistent with photosynthesis activity in leaves [44]. Therefore, it is possible that increment of Pn rate by the application of compost (Figure 4) might enhance stomatal conductance and the transpiration rate [35], transpiration, photosynthesis, chlorophyll biosynthesis and cell membrane integrity [42] in plants in the presence of Zn.
Compost application in soil increases microbial activity, nitrogen concentration which leads to increase grain yield [45]. Compost might be able to induce Zn function in plants to sustain the production of potato plants (Figure 5a), where compost plays a role in decreasing nutrients leaching from the sandy soil through increasing soil health such as water holding capacity. The foliar application of Zn increases the production of corn [32] indicates the efficiency of Zn in plants under different conditions [46]. Zn induces size of fruit through enhancing carbonic enzymes, which present in photosynthetic tissues, and increase chlorophyll biosynthesis [47]. Likewise, the weight of tuber increased due to the utilization of Zn when it was applied as a foliar spray (Figure 5b).Utilization of Zn increases the production of tubers, weight of tuber and performance in plant biosynthesis and the quality of sweet potato(Figure 6; [48]). Therefore, production of tuber is also attributed to the ash content, crude fibre and crude protein in tuber by the compost and Zn treatment (Figure 6).
In conclusion, sandy soil is detrimental to crop to decrease production due to retaining low characters of soil health. Zn-increased physiological functions were increased in the presence of compost. Compost helps improving soil health, which enhances plant parameters to sustain Zn-induced tuber yield in sandy soil.
AcknowledgementsThis work was supported by the Faculty of Bioresources and Food Industry, Universiti Sultan ZainalAbidin, Terengganu, Malaysia.
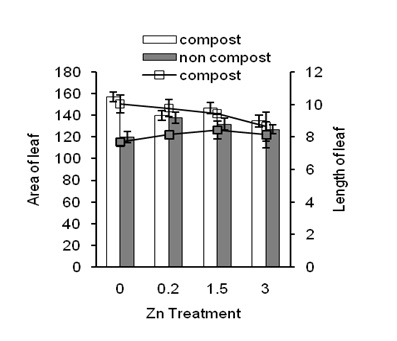
Figure 1: Effects of Zn on area of leaf (bar graphs) and length of leaf (line graphs) in the presence of compost (open bars and line) and absence of compost (closed bars and line) of sweet potato.
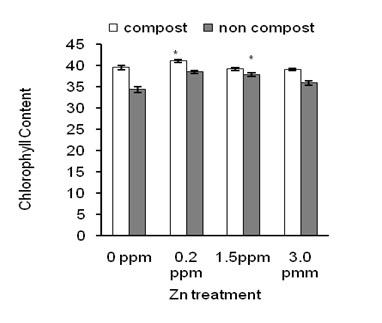
Figure 2: Effects of Zn on chlorophyll content in leaves of sweet potato in the presence of compost (open bars) and absence of compost (closed bars).
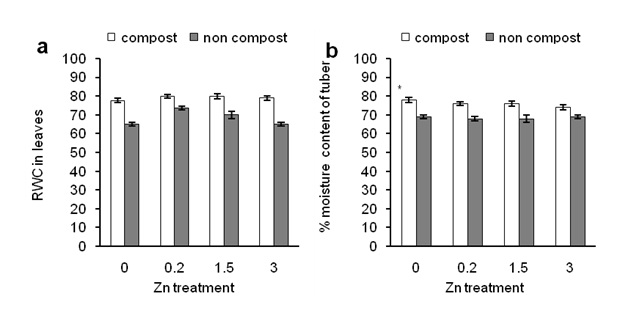
Figure 3: Effects of Zn on relative water content in leaves of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Effects of Zn on moisture content of tuber in the presence of compost (open bars) and absence of compost (closed bars).
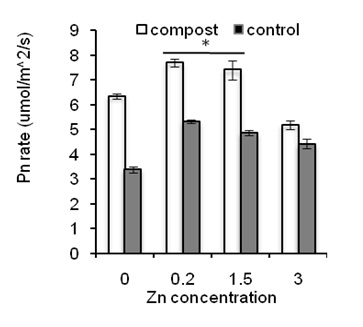
Figure 4: Effects of Zn on photosynthesis rate in leaves of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
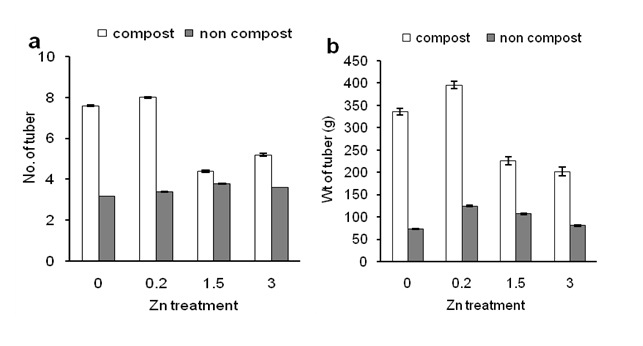
Figure 5: Effects of Zn on numbers of tuber of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Effects of Zn on weight of tuber of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
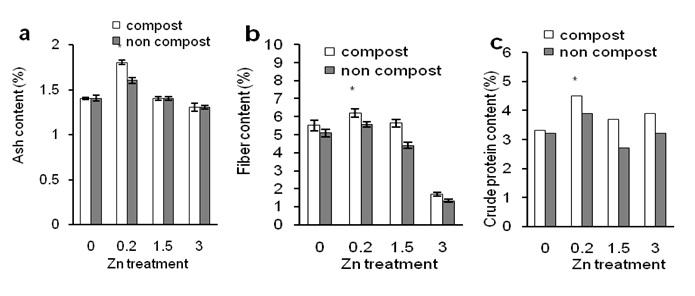
Figure 6: Effects of Zn on ash content of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Effects of Zn on fiber content of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Effects of Zn on crude protein content of sweet potato in the presence of compost (open bars) and absence of compost (closed bars). Error bars indicate standard deviation with 5 replicates.
Chat with us on WhatsApp