
Citation: Martins MLL et al. Sugarcane Bagasse and Passion Fruit Rind Flour as Substrates for Cellulase Production by Bacillus Sp. SMIA-2 Strain Isolated from Brazilian Soil. J Microbiol Biotechnol, 2017, 2(1): 000115.
*Corresponding author: Meire Lelis Leal Martins, Food Technology Laboratory, Universidade Estadual do Norte Fluminense, Avenida Alberto Lamego 2000, 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil, Email: meire@uenf.br
The application of wastes from the food processing industry as carbon sources in enzyme production processes reduces the cost of production, and helps solving problems related to their disposal. In this work, we demonstrated that the use of sugarcane bagasse as cellulosic substrate, in combination with passion fruit rind flour, as co-substrate and corn steep liquor as nitrogen source can be successfully employed for the production of avicelases (avicel-hydrolyzing enzymes) by Bacillus sp. SMIA-2. This would promote the use of these agricultural byproducts as novel and cost-effective culture media for the production of the enzyme. The maximum avicelase activity was obtained when Bacillus sp SMIA-2 was grown in liquid medium containing 0.625% (w/v) sugarcane bagasse, 0.625% (v/v) corn steep liquor and 075% (w/v) passion fruit peel flour for 168 hours.
Keywords: Bacillus Sp; Cellulase; Corn Steep Liquor; Passion Fruit Peel Flour; Sugarcane Bagasse
The complete enzymatic hydrolysis of cellulosic materials requires at least three different types of cellulases: endoglucanase (CM cellulase, EC 3.2.1.4), exoglucanase (Avicelase, EC 3.2.1.91) and β-D-glucosidase (β-D-glucoside glucohydrolase, EC 3.2.1.21) [1]. Among these types of cellulases, the avicelases seem to catalyze most of the bond-cleavages in the saccharification of crystalline cellulose and are usually the major component of cellulase preparations, especially in the case of current fungus-derived commercial enzymes [2]. Cellulases are used in different industrial applications such as pulp industry, food and animal feed, textile and detergent industry [3]. They are also applied in the production of bioethanol from lignocellulosic materials [4, 5].
The present cellulase toolbox is not sufficient to meet the industrial demand. For this reason, the major challenge in the development of economically feasible bioprocess for cellulase production is to identify the potential microorganisms, composition of media and the optimization of various process parameters that affect microbial growth and enzyme production [6]. The carbon source used in cultivations is one of the most important factors affecting the cost and yield of cellulase production. In view thereof, biosynthesis of cellulase has also been conducted on lignocellulosic materials, such as sugarcane bagasse [6], which is abundantly available at low cost as a byproduct from the sugar industry in Brazil [7]. Since the cellulose component of sugarcane bagasse is a structural polymer and is protected against enzymatic attack by the surrounding matrix of lignin and hemi cellulose, its pretreatment removes lignin, reduces crystallinity of cellulose, which may enhance cellulase production by microorganisms [8].
Employment of Response Surface Methodology (RSM) is considered the most promising tool in the optimization of process parameters for better yield [9,10]. In this work, the Central Composite Design (CCD) was adopted to maximize the production of cellulase by cultures of thermophilic Bacillus sp SMIA-2 containing sugarcane treated with alkali and corn steep liquor. Subsequently, it was evaluated the use of passion fruit rind flour as cosubstrate, as an alternative to the conventional rich medium.
Material and MethodsThe present study used a thermophilic Bacillus sp strain SMIA-2, previously isolated from a soil sample collected in the city of Campos dos Goytacazes, Rio de Janeiro, Brazil. Phylogenetic analysis revealed that the bacteria were closely related to Bacillus caldoxylolyticus and Bacillus sp strain AK1, and these three organisms exhibited levels of ribossomal DNA sequence homology of 94% [11].
Enzyme ProductionThe culture medium used in this work for cellulase production contained (g/L): KCl-0.3, MgSO4-0.5, K2HPO4- 0.87, CaCl2-0.29, ZnO-2.03x10-3, FeCl3.6H2O-2.7x10-2, MnCl2.4H2O-1.0x10-2, CuCl2.2H2O-8.5x10-4, CoCl2.6H2O- 2.4x10-3, NiCl3.6H2O-2.5x10-4 and H3BO3-3.0x10-4. In first set of experiments, sugarcane bagasse (SCB) treated with alkali (81.05% cellulose, 18.75% hemicellulose, 5.45% lignine) was used as a source of cellulose [12] and commercial corn steep liquor (Sigma Aldrich), as a nitrogen source. The SCB and corn steep liquor (CSL) concentrations were adjusted for each value according to central composite design (CCD), as presented in (Table 1).
The pH was adjusted to 7.2 with 1.0 M NaOH and the medium was sterilized by steam-autoclaving at 121oC, 1 atm for 15 minutes. The medium (50 mL in 250 mL Erlenmeyer flasks) was inoculated with 1 mL of an standard overnight culture (initial number of cells 104) and incubated at 50oC in an orbital shaker (Thermo Forma, Ohio, USA) operated at 150 rpm. Triplicate flasks were withdrawn at regular intervals and the contents were then centrifuged (HERMLEZ 382K, Wehingen, Germany) at 15,500 g for 15 min, at 4 ºC, and the cell free supernatant was used as crude enzyme preparation.
The effect of different co-substrates (0.5%, w/v) on avicelase secretion was assessed by supplementing the culture medium with passion fruit rind flour (obtained from a local market), apple pectin, fructose, glucose, xylose, lactose and cellobiose. In addition, the concentration of passion fruit rind flour (PFRF) in the culture medium ranged from 0.25% to 1.5% (w/v).
Enzyme AssayThe cellulolytic enzyme activities were determined using the dinitrosalicylic acid method [13], which measures reducing sugars. The reaction mixture containing 0.5 mL of 1% (w/v) avicel, PH-101 prepared in 10 mM sodium phosphate buffer, pH 7.5, and 0.5 mL of appropriate concentration of enzyme solution, was incubated at 70°C. After 10 min of reaction, 1 mL of dinitrosalicyclic acid reagent was added and boiled in water bath for 5 min. The resulting samples were then cooled to room temperature, and the absorbance was measured at 540 nm. One unit (U) of activity was defined as 1 μmole of glucose equivalent released per minute under the above assay conditions, by using a glucose standard curve. Appropriate controls were conducted in parallel with all assays. Enzyme blank containing 0.5 mL of 10 mM sodium phosphate buffer and 0.5 mL of 1% (w/v) substrate solution were run. To exclude the background of reducing sugars found in the enzyme supernatant from the results, a substrate blank containing 0.5 mL of 10 mM sodium phosphate buffer and 0.5 mL enzyme solution was also run. The absorbance of the enzyme blank sets and the substrate blank were subtracted from the absorbance of the activity assay. All of the samples were run in triplicate, while the blanks were run in duplicate.
Experimental Design and Statistical AnalysisThe surface-response methodology (SRM) was used to obtain a model for cellulase activity. To evaluate the effects of sugarcane bagasse and corn steep liquor concentration and culture time on the production of cellulase, a central composite design (CCD) 23 was constructed. The factorial planning had three central points and yielded a total of 17 treatments. The factors and levels studied are described in Table 1.
The results were analyzed using the Statistical software system, version 5.0. In this context, the F test was used as a validation criterion of statistical significance of the models obtained at a confidence level of 95%.
The optimization of condition was performed using CCD and surface-response was produced with fixed central points of 0.625% sugarcane bagasse, 0.625% corn steep liquor and incubation time of 168 hours. The experimental model can be expressed as follows:

Where bo, bi, bii and bij are the intercept terms, linear, quadratic coefficient and interactive coefficient, respectively, and xi and xj are coded independent variables.
Scanning Electron MicroscopyThe scanning electron microscopy (SEM) was used to obtain the degree of degradation of SCB and PFPF caused by Bacillus sp SMIA-2. Briefly, 72h bacterial cultures were harvested from culture media with 0.5% (v/v) CSL and 0.5% (w/V) of each of these substrates and fixed for 2 hours in 5% glutaraldehyde, 0.05 M sodium phosphate buffer, pH 7.1. Subsequently, the samples were washed 3 times in the same buffer and dehydrated in a graded alcoholic series (20, 40, 60, 70, 80, 90, 96 and 100%) for 15 min each. After dehydration, the samples were dried with CO2 using a BAL-TEC CPD 030 Critical Point Dryer and then mounted in an aluminum sample holder and covered with a layer of palladium in a Bal-tec SCD 050 on atmosphere of argon for 120 seconds. Thereafter, the samples were observed on a ZEISS DSEM 962 scanning electron microscope (Lichtenstein, Germany) at the UENF Center for Bioscience and Biotechnology.
ResultsInitially, a central composite design (CCD) 23 was constructed to evaluate the effects of sugarcane bagasse and corn steep liquor concentration and culture time on avicelase production by Bacillus sp SMIA-2. According to Table 1, a variation in avicelase activity was observed from 0.0 (Treatments 4, 7, 8 and 12) to 2.73 U.mg protein- 1 (Treatment 16). Comparing the parameters studied in these experiments, it seems that the maximum avicelase activity was obtained when Bacillus sp SMIA-2 was grown in liquid medium containing 0.625% (w/v) SCB and 0.625% (v/v) CSL for 168 hours.
The statistical significance of the model equation was assessed by an F-test (ANOVA) and the data are shown in Table 2. An equation for avicelase activity (Equation 2) was developed based on a regression analysis of the following experimental data:

The outcome of ANOVA analysis revealed that the adjusted model was significant, according to the analysis of the F test. The regression model for avicelase production was highly significant (p < 0.05), with a satisfactory value of determination coefficient (R2 = 87.98). The response surface was produced according to Rodrigues and Lemma [14].
The response surface and contour plot figures obtained by the analysis of the experimental data of CCD showed a relationship between two variables at time. The nonexplicit variables were fixed at the central point (level 0) for the surface construction. As showed in Figure 1 an initial increase in incubation time with simultaneous increase in SCB concentration, when keeping the CSL concentration constant at 0.625% (v/v), resulted in an increase of avicelase production. However, increasing SCB concentration beyond this limit decreased the avicelase production. In addition, an initial increase in incubation time, with simultaneous increase in CSL concentration, at constant SCB concentration (0.625%, w/v), increased avicelase production. However, when the concentration was higher than 0.625% (w/v), the enzyme production decreased. Finally, an increase in SCB concentration with simultaneous increase in CSL, with the fixed coded value of incubation time (168 h) led to an initial increase in avicelase production until reaching the optimal enzyme, 0.625% (w/v), for both ingredients, which was followed by a decrease.
The addition of different co-substrates to the optimized medium improved avicelase production. The supplementation of fructose, xylose, lactose and cellobiose had little effect on avicelase production, while glucose, pectin and PFRF enhanced enzyme production. Among those, PFRF provided the highest levels of cellulase activity, around 30%, compared to the control.
Figure 2 shows the effect of different incubation periods on the production of avicelase by Bacillus sp SMIA-2 grown in the optimized culture medium supplemented with PFRF (0.5%, w/v). The maximum avicelase activity (3.6 U.mgprotein-1) was obtained after 168 h of incubation culture. The visualization of Bacillus sp SMIA-2 cells growing on SCB and PFRF was performed using scanning electron microscopy (SEM) studies (Figure 3). Bacillus sp SMIA-2 attached firmly to both SCB and PFRF, which is an important characteristic in the degradation process of these substrates. In addition, the images provide qualitative observations of the cell population. Despite the difficulty in differentiating microbial cells from SCB, the number of cells grown in this insoluble substrate (Figure 4A) was much lower compared with PFRF (Figure 4B).
In order to find the best concentrations of PRFR for avicelase production, different amounts were used in the culture medium, ranging from 0.25% to 1.5% (w/v). The activity of the enzyme increased at PRFR concentrations between 0.25% and 0.75% (w/v) and then dropped at higher concentration levels (Figure 5). Thus, the medium containing 0.625% (v/v) CSL, 0.625% (w/v) SCB and 0.75% (w/v) PRFR was considered the most effective for avicelase production by Bacillus sp strain SMIA-2.
DiscussionThe optimization study of avicelase (avicel-hydrolyzing enzymes) production by thermophilic Bacillus sp SMIA-2 showed that the amounts of SCB and CSL in the culture medium and fermentation time strongly affected the activity of this enzyme. The microbial production of cellulases is usually induced by SCB. Therefore, celluloserich wastes are exploited as abundant and inexpensive growth substrates to obtain cellulases [8]. CSL is a source of amino acids, vitamins and metal ions and has been successfully used in culture media for cellulase production [15,16] instead of meat and yeast extracts, which are expensive nitrogen sources. The highest avicelase activity (2.73 U.mg protein-1) was obtained when Bacillus sp SMIA-2 was grown in liquid medium containing 0.625% (w/v) treated SCB and 0.625% (v/v) CSL for 168 hours, which was the central point chosen in the experimental design approach used in this study. The central point was properly chosen based on previous studies carried out in our laboratory [17]. Thus, the experimental design approach used in this study was found to be efficient for the rapid optimization of the process parameters for cellulase production by Bacillus sp SMIA-2. Several published works have employed the response surface methodology as a tool for optimization of the cellulase production process. However, comparison between the activities obtained with Bacillus sp. SMIA-2 and the published literature was hindered by the different definitions of enzymatic activity and different levels of enzyme purity used.
Cirigliano et al. [16] optimized the production of endoglucanase by Streptomyces misionensis strain PESB- 25 using response surface methodology (RSM). A peak of endoglucanase accumulation (1.01 U x [mL.sup.-1]) was observed in a medium with SCB 1.0% (w/v) and CSL 1.2% (w/v), within three days of cultivation. The maximum CMCase activity (2040 IU/L) was achieved when Bacillus sp. JS14 was grown on a wheat bran concentration of 400 g/L, pH 6.5, at 40 0C and incubation period of five days [9]. The optimization of cellulase production by Bacillus amyloliquefaciens UNPDV-22 using RSM resulted in a 96% increase in the enzyme activity over the control of nonoptimized basal medium. Optimum cellulase production of 13 U/mL was obtained at 42.24 °C, pH of 5.25, and inoculum size of 4.95% (v/v) in a fermentation medium containing wheat bran, soybean meal and malt dextrin as major nutritional factors [10]. The optimum parameters for cellulase production by Bacillus pumilus EWBCM1 using RSM based on CCD model were galactose of 1.0 g/L, malt extract of 0.5 g/L and incubation time of 72 hrs. Optimization with coded factor provided the maximum cellulase production observed by the model, 0.5751 IU/ml [18].
A successful and significant improvement (1.3-fold) in avicelase production by Bacillus sp. SMIA-2 was obtained when the optimized culture medium was supplemented with 0.5% (w/v) PFRF. It demonstrates the benefits of using co-substrates for increasing enzyme activity. Simple sugars have often been reported to work as repressors of enzyme activity. However, the mixture of substrates, including simple sugars as co-substrates, had been evaluated for the production of crude extract containing the enzyme pools with cellulolytic activity [19]. Muthuvelayudham and Viruthagiri [20] analyzed the effect of different carbon sources such as glucose, lactose and xylose on submerged fermentation, using isolates of T. reesei, and verified that the combination of cellulose and xylose led to the best level of cellulase production.
The highest avicelase activity (3.6 U.mgprotein-1) secreted by Bacillus sp. SMI-2 grown in liquid medium containing SCB (0.625%, w/v), CSL (0.625%, v/v) and PRFR (0.5%, w/v) was reached after 168 h of culture time. This incubation period was longer than that reported for maximum avicelase production by Bacillus sp SMIA-2 grown on untreated SCB [17] and shorter than that reported for cellulase production by Bacillus sp [21].
The presence of 0.75% (w/v) PRFR supported maximal avicelase (4.0 U.mgprotein-1). Passion fruit processing generates a substantial amount of residues, including peel, which is an environmental problem [22]. Its rich composition might supply nutrients that work as growth enhancers for microbial processes. The peel of passion fruit is rich in fiber, minerals, and especially pectin. In fact, qualitative observations from SEM images of Bacillus sp SMIA-2 grown on SCB or PRFR showed the potential of PFRF to be used as co-substrate to increase cell population. According to Maurosa et al. [23], measurements of bacterial growth on insoluble substrates are difficult for several reasons. For example, viable counts on plates of agar media are complicated by the propensity of cellulose-decomposing cells to adhere to the substrate, which hinders the accurate dilution of cultures. These authors described a dual-staining procedure to visualize and enumerate cells in the presence of insoluble plant biomass substrates.
In conclusion, SCB combined with PFRF can be successfully used for the production of avicelase (avicelhydrolyzing enzymes) by Bacillus sp. SMIA-2, opening perspectives for the use of these agricultural byproducts as novel and cost-effective culture media for the production of the enzyme.
AcknowledgmentsThe authors thank the FAPERJ — Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro for financial support and Dr Fábio Olivares for providing support in analyzing the samples using SEM.
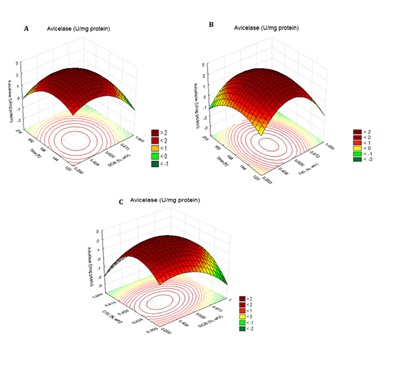
Figure 1: Three-dimensional response surface plot for: Effect of incubation time and SCB concentration on avicelase production by Bacillus sp SMIA-2 at a constant CSL concentration (A); Effect of incubation time and CSL concentration on avicelase production by Bacillus sp SMIA-2 at a constant SCB concentration (B); Effect of SCB and CSL concentration on avicelase production by Bacillus sp SMIA-2 at a constant incubation time (C). Dark red color indicates high activity, while green and yellow color indicates low avicelase activity.
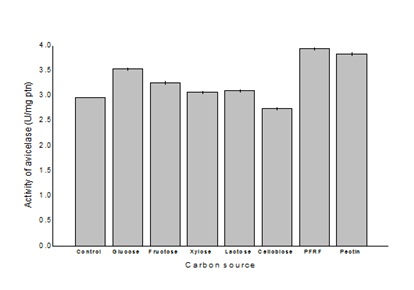
Figure 2: Effect of different fermentable sugars on avicelase production by Bacillus sp SMIA-2 grown in submerged culture containing 0.625% (w/v) SCB and 0.625% (v/v) CSL at 50ºC for 168 h.
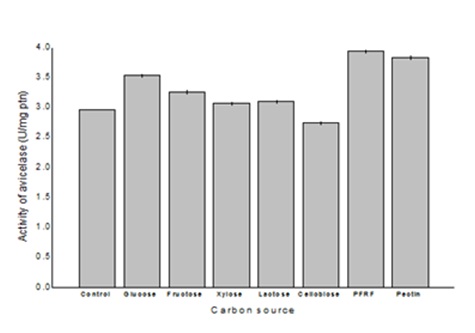
Figure 3: Effect of incubation time on avicelase production by Bacillus sp SMIA-2 grown in submerged culture containing 0.625% (w/v) SCB and 0.625% (v/v) CSL and supplemented with 0.5% (w/v) PRFR at 50ºC.
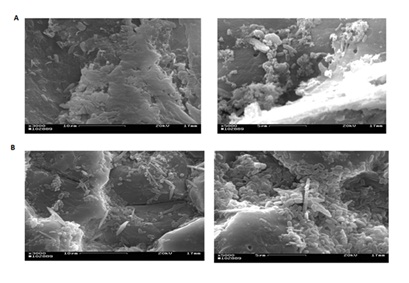
Figure 4: Scanning electron microscopy (SEM) images showing cells of Bacillus sp SMIA-2 grown on 0.5% (v/v) CSL and 0.5% (w/v) SCB (A) or 0.5% (v/v) PFRF (B) at 50°C for 72 hours.
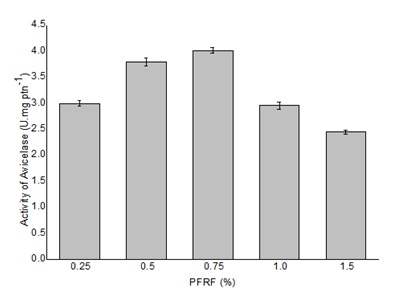
Figure 5: Effect of different concentrations of PRFR on avicelase production by Bacillus sp. strain SMIA-2 grown in submerged culture containing 0.625% (w/v) SCB and 0.625% (v/v) CSL at 50 °C for 168 h. Results represent the means of three separate experiments, and bars indicate ± 1 standard deviation.
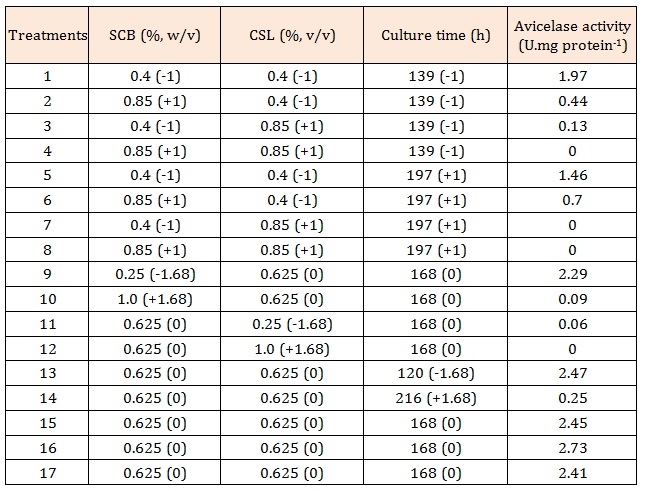
Table 1: Matrix of CCD 23 (real and coded values) used and its response (avicelase activity).
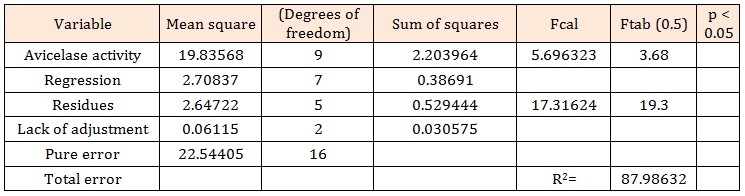
Table 2: ANOVA for the variables of response surface quadratic model for avicelase production
Chat with us on WhatsApp