
Citation: Asif Ml. A Mini Review on Antibacterial and Anti-Fungal Activity of PyrazoloPyridazine and Pyrazolo-Cinnoline Derivatives. J Pharm Res 2017, 1(3): 000117.
*Corresponding author: Mohammad Asif, Department of Pharmacy, GRD (P.G) IMT, Dehradun, India, 248009, Tel: +919897088910, Email: aasif321@gmail.com
The in vitro antibacterial and antifungal potential of pyrazolo-pyridazine derivatives (4a-l), (4’a-l) and (4’’a-m). The 3,5- Disubstitutedphenyl-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c]pyridazine derivatives were prepared by multi-step synthesis. Reaction of benzoyl, touloyl and p-chlorobenzoyl propionic acid (1a-c) with hydrazine hydrate gave (6-phenyl, 6-touloyl and p-chlorophenyl)-tetrahydropyridazin-3-one (2a-c). This on treatment with aryl-aldehydes resulted in the formation of pyridazinone (3a-l), (3’a-l) and (3’’a-m). In the final step, the pyridazinones (3a-l), (3’a-l) and (3’’a-m) were reacted with hydrazine hydrate to furnish the compounds 4 (a-l), (4’a-l) and (4’’a-m). These compounds were screened for in vitro antibacterial and antifungal activities. Compounds 4i, 4’f, 4’’d, 4’’e and 4’’f was found to be significant in its action against Gram positive and Gram negative bacteria, whereas compound 4f, 4’c, 4’’c and 4’’d exhibited potent antifungal activity. Compound 4’’l, was found to have significant action against Gram positive and Gram negative bacteria, whereas compound 4’’k, exhibited potential antifungal activity. Other series of Pyridazine derivatives were synthesized by diazotization of substituted anilines followed by FriedelCrafts acylation and coupling to form corresponding hydrazones which on intra-molecular cyclization forms 3-acetyl-substitutedbenz-pyridazine-4(1H)-one. Further, condensation reaction by treatment with hydrazine hydrate yields the 3’-methyl-substituted-pyrazolo [4,3-C]Cinnolines. All cinnoline derivatives were showed good anti-fungal activity against various pathogenic bacteria and fungi. The compound 5e was found to be safe and moderate drug in comparison to standard drug.
Keywords: Antimicrobial; Cinnoline; Pyrazole; pyridazine; Antibacterial; Antifungal; Fused
Pyridazine, six membered nitrogen containing heterocyclic ring, plays an important role in pharmaceuticals particularly in the field of medicinal chemistry. Pyridazine ring is a part of the structures of a number of drugs available in the market like hydralazine, minaprine, cefozopran, pipofezine, etc. Pyridazine derivatives have been reported to possess various pharmacological activities including antimicrobial, analgesic, anticancer, antifeedant, antitubercular, antidiabetic, antifungal, antihypertensive, antiplatelet, anticonvulsant, anti-HIV, antiasthma, anti-inflammatory, phosphodiesterase (PDE) inhibitors, cyclooxygenase (COX) inhibitors, antipyretic, insecticidal, neurological activity like anti anxiety and depressant, and intermediates for drugs synthesis, agrochemicals and other anticipated biological properties [1-7]. Similarly, another heterocycle, pyrazole, is five-membered nitrogen containing heterocyclic ring systems. Pyrazole derivatives have also been reported to possess potential biological activities including antibacterial and antifungal actions. On the other hand, pyrazole derivatives are well established in the literatures as important biologically effective heterocyclic compounds. They act as antiinflammatory, antipyretic, antimicrobial, antiviral, antitumor, anticonvulsant, antihistaminic, antidepressant, and potential agents against A549 lung cancer cells. Recently, some arylpyrazoles were reported to have nonnucleoside HIV-1 reverse transcriptase inhibitor activity. Moreover, some pyrazolopyridazines are good antiinflammatory agents, potent inhibitors of glycogen synthase kinase-3 (GSK-3) and selective cyclin dependent kinase (CDK4) inhibitors. In view of the antimicrobial activities exhibited by pyridazines and pyrazoles, it was thought worthwhile to study their fused derivatives as potential antibacterial and antifungal agents [8-10].
In recent year we developed new synthetic approaches for the construction of biologically active heterocyclic compounds. The goal of the present work was directed to synthesize novel derivatives of pyrazolo-pyridazine to evaluate their antibacterial and antifungal activities. In view of the antimicrobial activities associated with pyridazine and pyrazole derivatives, it was thought worthwhile to study their fused derivatives as antibacterial and antifungal agents. Thus, several pryrazolopyridazine derivatives have been synthesized and evaluated for their antibacterial and antifungal actions against six microbial strains. Keeping in mind, the wide array of antimicrobial activities exhibited by pyridazines and pyrazoles, it was thought to develop new fused derivatives comprising of pyrazole and pyridazine in the same structure as potential antibacterial and antifungal agents [11-15].
General MethodThe synthesis of pyridazine derivatives involved three steps, exemplified by the synthesis of compounds 4. The synthesis of intermediates 2 and 3 and target compounds 4 was performed by adopting the synthetic protocol illustrated in Scheme 1.
Compound 2a-c, (6-aryl-2,3,4,5-tetrahydropyridazin-3- one) was synthesized. Its synthesis involved the reaction of aroylpropionic acid (1a-c) with hydrazine hydrate. This compound 2a-c was further used for the preparation of compounds 3(a-l); (4E)-4-substitutedarylidene-6- substitutedaryl-4,5-dihydropyridazin-3(2H)-ones. Finally using 3(a-l), compounds 4 (a-l); 3,5-disubstituted aryl- 3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c]pyridazine were prepared [11-14].
Synthesis of 4-substituted-benzylidene-6-(4- methylphenyl)-4, 5-dihydro-pyridazin-3(2H)- one (3)A mixture of compound 2 (0.01 mol) and appropriate aryl-aldehydes (0.01 mol) in ethanol (20 ml) was taken in a round bottom flask. To this, piperidine (1 ml) was added with stirring and then the reaction mixture was refluxed for 5-8 h. On completion of reaction, the contents were cooled and then poured onto crushed ice. A solid mass separated out, which was filtered, washed with water, dried, and recrystallized from methanol.
Synthesis of 3-substituted-phenyl-5-(4- methylphenyl)-3,3a,4,7-tetra-hydro-2Hpyrazolo [3,4-c]pyridazine (4)Ethanolic solution of compounds 3a-g (0.01mol) was taken in a round bottom flask. To this, hydrazine hydrate (0.02mol) was added and the resulting reaction mixture was refluxed on steam bath for 8-10 h. After completion of reaction, the contents were concentrated, cooled and poured onto crushed ice. The separated solid was filtered, washed with water, dried, and recrystallized from methanol.
The 3,5-disubstitutedaryl-3,3a,4,7-tetrahydro- 2Hpyrazolo[3,4-c]pyridazine 4(a-l): 3,5-diphenyl-3,3a, 4,7-tetrahydro-2Hpyrazolo[3,4-c]pyridazine(4a), 3-(2- chlorophenyl)-5-phenyl-3,3a,4,7-tetrahydro-2Hpyrazolo[3,4-c]pyridazine (4b), 3-(3-chlorophenyl)-5- phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo [3,4- c]pyridazine(4c), 3-(3-bromophenyl)-5-phenyl-3,3a, 4,7- tetrahydro-2H-pyrazolo[3,4-c] pyridazine (4d), 3-(4- bromophenyl)-5-phenyl-3,3a,4,7-tetrahydro-2Hpyrazolo[3,4-c]pyridazine (4e), 3-(4-fluorophenyl)-5- phenyl-3,3a,4,7 tetra-hydro-2H-pyrazolo[3,4-c]pyridazine (4f), 4-(5-phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo [3,4- c]pyridazin-3-yl)phenol(4g), 3-(4-methoxyphenyl)-5- phenyl-3,3a,4,7-tetra hydro-2H-pyrazolo[3,4- c]pyridazine(4h), 3-(3,4-dimethoxyphenyl)-5-phenyl- 3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c]pyridazine(4i), 2- methoxy-4-(5-phenyl-3,3a,4,7-tetrahydro- 2Hpyrazolo[3,4-c]pyridazin-3-yl)phenol (4j), 3-(4- methylphenyl)-5-phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo [3,4-c]pyridazine (4k), 4.N,N-dimethyl-4-(5-phenyl- 3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c] pyridazin-3- yl)aniline (4l) [1].
The 3-substituted-phenyl-5-(4-methylphenyl)-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c]pyridazine (4’a-g): 3,5- Diphenyl-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazine (4’a), 3-(2-Chlorophenyl)-5-phenyl-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c]pyridazine (4’b), 3-(4- Chlorophenyl)-5-phenyl-3,3a,4,7-tetrahydro-2Hpyrazolo[3,4-c]pyridazine (4’c), 3-(4-Fluorophenyl)-5- phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c]pyridazine (4’d), 4-(5-Phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazin-3-yl)phenol (4’e), 3-(4-Methoxy phenyl)-5- phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c]pyridazine (4’f), 3,5-Bis(4-methyl phenyl)-3,3a,4,7-tetrahydro-2Hpyrazolo[3,4-c]pyridazine (4’g) 3-(3-Bromophenyl)-5-(4- methylphenyl)-3,3a,4,7-tetra hydro-2H-pyrazolo[3,4- c]pyridazine (4’h), 3-(4-Bromophenyl)-5-(4- methylphenyl)-3,3a,4,7-tetra hydro-2H-pyrazolo[3,4-c] pyridazine (4’i), 5-(3,4-Dimethoxyphenyl)-3-(4- methylphenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazine (4’j), 5-(4-Hydroxy-3-methoxyphenyl)-3-(4- methyl-phenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo [3,4-c] pyrida-zine (4’k), 4-(5-(4-Methylphenyl)-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c] pyridazin-3-yl)-N,Ndimethyl benzenamine (4’l) [2,4].
The 3-substituted-phenyl-5-(4-chlorophenyl)-3,3a,4,7- tetrahydro-2Hpyrazolo[3,4-c]pyridazine (4’’a-h): 5-(4- Chlorophenyl)-3-phenyl-3,3a,4,7-tetrahydro-2Hpyrazolo[3,4-c]pyridazine (4’’a), 3-(2-Chlorophenyl)-5-(4- chlorophenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazine (4’’b), 3,5-bis(4-Chlorophenyl)-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c]pyridazine (4’’c), 5-(4- Chlorophenyl)-3-(4-fluorophenyl)-3,3a,4,7-tetrahydro- 2H-pyrazolo[3,4-c]pyridazine (4’’d), 4-{5-(4- Chlorophenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazin-3-yl}phenol (4’’e), 5-(4-Chlorophenyl)-3-(4- methoxyphenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazine (4’’f), 5-(4-Chlorophenyl)-3-(4- methylphenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c] pyridazine (4’’g), 4-{5-(4-Chlorophenyl)-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c]pyridazine-3-yl}-N,Ndimethyl benzenamine (4’’h), 3-(3-Bromophenyl)-5-(4- chlorophenyl)-3,3a,4,7-tetra hydro-2H-pyrazolo[3,4- c]pyridazine (4’’i), 3-(4-Bromophenyl)-5-(4- chlorophenyl)-3,3a,4,7-tetra hydro-2H-pyrazolo[3,4-c] pyridazine (4’’j), 5-(3,4-Dimethoxyphenyl)-3-(4- chlorophenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazine (4’’k), 5-(4-Hydroxy-3-methoxyphenyl)-3- (4-chlorophenyl)-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4- c]pyridazine (4’’l) and 4-(5-(4-Chlorophenyl)-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c]pyridazin-3-yl)-N,Ndimethyl benzenamine (4’’m) [3,4].
Synthesis of Ethyl 3-oxo-2-(2-substituted phenyl hydrazinylidene) butanoateVarious substituted anilines (0.39mol) were dissolved in a mixture of concentrated Hydrochloric acid (15ml) and water (15ml) and cooled to 0-5°C in ice bath; it was then added to a cold saturated solution of sodium nitrite (0.58mol) with constant stirring. The diazonium salt thus formed was filtered into a cooled solution of ethyl acetoacetate (0.39mol) in ethanol and sodium acetate in water (to make it alkaline).The solid was collected and recrystallized from methanol [15].
Synthesis of 3-Acetyl Cinnoline-4(1H)-one derivativeTo Ethyl 3–oxo-2-(2-phenyl hydra-zinylidene) butanoate (0.01mol) was added anhydrous Aluminium chloride (0.02mol). Chlorobenzene (30ml) was added in order to dissolve the solids and the mixture was then refluxed for 1hr.the complex formed was decomposed with concentrated hydrochloric acid (30 mL) and diluted with cold water. The product was filtered, washed with water, dried and recrystallized from methanol [15].
Synthesis of Pyrazolo CinnolinesA mixture of Ethyl 3-oxo-2-(2- phenyl hydrazinylidene) butanoate (0.005mol) and hydrazine hydrate/phenyl hydrazine (0.02mol) in ethanol was refluxed for 3 hrs. The product formed was collected and recrystallized from ethanol [15].
7-Chloro-3-methyl-1H-pyrazolo [4,3-c]Cinnoline (5a), 7-Chloro-3-methyl-1phenyl-1H-pyrazolo[4,3-c]Cinnoline (5b), 7-Fluoro-3-methyl-1phenyl-1H-pyrazolo[4,3- c]Cinnoline (5c) 3-Methyl-7-nitro-1H-pyrazolo [4,3-c]Cinnoline (5d), 3- Methyl-7sulphonamido-1H-pyrazolo [4,3-c]Cinnoline (5e), 3-Methyl-7-sulphonnamido-1phenyl -1Hpyrazolo[4,3-c]Cinnoline (5f)
Antibacterial activityAntibacterial activity of the compounds was determined by adopting cup plate method. The synthetic compounds diluted with dimethyl sulfoxide (DMSO) at three different concentrations (50, 100, and 200 μg/mL) were added to each well separately. After the stipulated period of 24 h, the activity of compounds in terms of zone of inhibition was observed against two Gram-positive: Staphylococcus aureus (S. aureus) and Micrococcus luteus (M. luteus), and two Gram-negative microbial strains; Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae). Ampicillin and Chloramphenicol antibiotics were used as positive control for the comparison purpose. Antibacterial activity of the synthesized compounds is reported in Tables 1, 3 and 4 [11-14].
Antifungal activityThe Sabouraud agar medium (dextrose 4%, peptone 1%, and agar 1.5%) was used for determining antifungal activity of the compounds. After duration of 2 h, the two fungal strains; Candida albicans (C. albicans) and Cryptococcus neoformans (C. neoformans) were inoculated on the surface of Petri plates separately. Then 0.1 mL of each standard (100 μg/mL) and test compounds (100, 250 and 500 μg/mL) prepared by dissolving in DMSO was added into cups. Growth and zones of inhibition (in mm) were recorded. Fluconazole and Griseofulvin drugs were used as positive control for the comparison purpose. The antifungal activity of synthesized compounds is tabulated in Tables 2 and 5.
Anti Fungal ScreeningDisc diffusion method: Sabourands dextrose broth medium was prepared and transferred into sterile Petri plates aseptically (thickness of 5-6mm). Standardized fungal inoculums of Aspergillus niger, Aspergillus fumigatus, Candida albicans, Monascus purpureus. The sample impregnated discs (10μg /disc) in dimethyl sulphoxide and standard clotrimazole 10μg/disc were placed on the inoculated agar medium. After the incubation diameter of zone of inhibition produced by the sample were measured [15].
Results and DiscussionThe compounds were screened for their antibacterial activity against S. aureus, M. luteus, E. coli and K. pneumoniae by cup plate technique in nutrient agar at concentrations of 100 and 200μg/ml (Table 1A-3A), Ampicillin and Chloramphenicol as standard drugs for comparison. Most of the compounds have comparable activity against the tested bacterial strains. Compounds 4i and 4l were found to be the highly active against both Gram positive and Gram negative bacterial strains. It was observed that increase in the number of electron releasing group(s) resulted in enhancement in the activity against both types of strains. Compound bearing methoxy groups (4i) exhibited best activity against all the four strains. The title compounds were also evaluated for their antifungal activity against C. albicans and C. neoformans using cup plate method at concentrations of 250 and 500μg/ml (Table 1B-3B). The zone of inhibition (mm) of each compound was determined and compared with the standard drug, Fluconazole. The compounds bearing electron withdrawing group exhibited better activity against both the strains. Further, compound bearing fluorine group (4f) was found to be the most active antifungal agent.
The synthesized compounds (4’a-g), (Table 1A), most of the compounds have comparable activity against the tested bacterial strains. Compounds 4’c and 4’f were found to be the highly active against both Gram positive and Gram negative bacterial strains. Compound bearing methoxy group (4’f) exhibited best activity against all the four strains. Compounds 4’d and 4’g showed moderate type of activity against the bacteria. The compounds bearing electron withdrawing group (4’c & 4’d) exhibited better activity against both the strains. Further, compound bearing chlorine group (4’c) was found to be the most active antifungal agent. Rests of the compounds were moderate in their antifungal action (Table 1B). The title compounds (4’’a-h) (Table 2A), most of the compounds have comparable activity against the tested bacterial strains. Compounds 4’’d, 4’’e and 4’’f were found to be the highly active against both Gram positive and Gram negative bacterial strains. Compounds bearing fluoro, hydroxyl or methoxy group exhibited very good activity against all the four strains. The results indicated that compounds bearing electron withdrawing group at Para-position (4’’c & 4’’d) exhibited better activity against both the strains. Rests of the compounds were moderate in their antifungal action (Table 2B). Further, compound bearing fluorine group (4’’d) was found to be the most active antibacterial and antifungal agent. Both Ampicillin and Chloramphenicol showed broad spectrum of antibacterial activity against all the four microbes at the dose of 50μg/mL. All the title compounds (4’’a-m) except 4’’k exhibited moderate to good antibacterial activity at a concentration of 100μg/mL. It is evident from the results of antibacterial evaluation, that most of the compounds have comparable activity against the tested bacterial strains. The maximum inhibition of microbial growth was noted at 200 μg/mL concentration and compounds 4’’l and 4’’k were found to be the highly active against both Gram-positive and Gram-negaitive bacterial strains. Compound 4’’l, in which 4-chlorophenyl is attached to pyridazine ring and 4-hydroxy, 3-methoxy phenyl substituent is connected to pyrazole core, exhibited the best activity against all the four strains. Compounds 4’’m and 4’’i showed moderate type of activity against the bacteria (Table 3A). It was quite surprising that compounds 4’h, 4’i did not show any activity against M. luteus even at the highest concentration. Similarly, K. pneumoniae was found to be resistant to 4’k and 4’l at a concentration of 200 μg/mL concentration. The compounds 4’h, 4’i, 4’k and 4’l have common structural feature i.e., these compounds bear electron donating groups on both phenyl rings, however, the most active compounds 4’’l and 4’’k have an electron withdrawing group (-Cl) on phenyl ring attached to pyridazine nucleus. Interestingly, compound 4’’i and 4’’j which contain electron withdrawing groups (-Cl, -Br) on both phenyl rings are less active than the other compounds in series (4’’k-m), which have one electron withdrawing group and one electron donating group on phenyl rings. Thus it could be concluded that presence of electron donating group such as methyl on phenyl ring at C-5 position of fused bicyclic ring system leads to decrease in antibacterial activity. The zone of inhibition (mm) of each compound was determined and compared with the standard drug, Fluconazole and Griseofulvin. A concentration dependent anti-fungal activity was noted for the tested compounds. All the compounds were inactive against the fungal strains at the lowest concentration of 100 μg/mL barring 4’’h and 4’’i. The result clearly indicated that compounds bearing electron donating groups (4’i and 4’’i) exhibited better activity against both the fungal strains. Further, compound bearing two electron withdrawing groups (4’’j) was found to be the most active antifungal agent and its activity was comparable with the standard drugs (Table 3B). The rest of the compounds were moderate in their antifungal action [11-14].
The Pyrazolo pyridazine derivatives (5a-5f) have been synthesized by the Intramolecular cyclization of the corresponding phenyl hydrazones acquired from diazotization of substituted Anilines followed by Friedel crafts acylation with ethyl aceto acetate in aqueous ethanolic solution containing sodium acetate and by reaction with hydrazine. All the compounds (5a-5f) were subjected for biological screening as antimicrobial agents by disc diffusion assay. Evaluation of the results from anti-bacterial and anti-fungal studies showed that synthesized Pyridazine derivatives were exhibits moderate to good antibacterial and anti-fungal activity as compared to standard (Table 4A). The MIC of the synthesized compounds against C. albicans, Aspergillus fumigates, Monascus purpureus was determined by serial diluton method, was found to be in the range of 1.2 to 3μg/ml and antibacterial in the range of 1.2 to 5.2μg/ml (Table 4B) [15].
These pathogenic microbes are common cause of bacterial infections in humans affecting skin, lungs, nose, mouth, GIT and other organs. They are also routinely detected in very young, very old and people suffering from some other diseases such as cancer [23]. K. pneumoniae is the most common cause of hospital acquired respiratory tract and premature intensive care infections. S. aureus and E. coli can be grown and cultured easily and inexpensively in a laboratory setting, and has been intensively investigated for many decades [16-18].
In recent years, the incidence of systemic bacterial and fungal infections is on rise due to an increase in the number of immune-compromised hosts. Immunosuppression due to malignancy, immunesuppressive therapies, HIV-infection, broad-spectrum antimicrobial treatment and age, as well as invasive procedures and mucosal barriers places patients at risk for microbial infections. The increasing incidence of resistance to a large number of antibacterial agents is becoming a major concern. Currently, a small number of antifungal agents are available, and all have some drawbacks regarding their spectrum, toxicity, tissue distribution, and high cost [19-22]. These observations place new emphasis on the need of as well as search for alternative new and more effective antimicrobial agents with a broad spectrum activity. In view of the antimicrobial activities exhibited by nitrogen containing heterocyclic such as pyridazines and pyrazoles, it was thought worthwhile to study their fused derivatives having pyrazole and pyridazine in the same structure as potential antibacterial and antifungal agents. We have previously reported the antibacterial and antifungal activity of some pryazolo-pyridazine derivatives possessing an unsubstituted phenyl and a meta or Para substituted phenyl group that respectively adjacent to pyridazine and pyrazole moiety of the fused bicyclic ring system. It was observed that the compounds which were bearing electron donating groups showed potent antibacterial activity, while compounds having electron withdrawing groups exhibited excellent antifungal activity. Therefore, we thought to synthesize a library of pyrazolo-pyridazine derivatives bearing different substituents on the two phenyl rings in order to study the structure activity relationship by evaluating their antibacterial and antifungal activities against some selected pathogenic microbes. The heterocyclic nitrogen compounds like Pyrazolo-benzpyridazine derivatives have a crucial role in synthetic drugs and biological processes [22-24]. Cinoxacin is a cinnoline [Benzpyridazine] isosteric analogue of the Quinoline antibacterials used for urinary tract infection. Most of azoles are used as effective anti-fungal agents. This incited us in the synthesis of new congeners as analogs of 4- methyl Benzo pyridazine and fusing pyrazole hoping to get more potent anti bacterial and anti fungal activity. It has been designed and synthesized some novel compounds which take account of both the advantage of pyrazole and Benzo Pyridazine [Cinnoline] nucleus in the single molecule [11-15].
ConclusionThe 3-substituted-aryl-5-(4-sustitutedaryl)-3,3a,4,7- tetrahydro-2H-pyrazolo[3,4-c] pyridazine were successfully synthesized through multistep synthesis. The potential of fused heterocyclic compounds pyrazolopyridazines 4(a-l) as antimicrobial agents against four bacterial and two fungal strains. It is concluded that compound bearing two methoxy groups (4i) appeared most active against Gram positive and Gram negative bacteria, whereas compound bearing electron withdrawing group (4f) exhibited potential antifungal activity. These compounds could be further derivatized to get even better antimicrobial agents. Compound bearing methoxy group (4’f) appeared most active against Gram positive and Gram negative bacteria, whereas compound bearing electron withdrawing group (4’c & 4’d) exhibited potential antifungal activity. Compounds bearing fluoro, hydroxyl or methoxy group (4’’d, 4’’e and 4’’f) were highly active against all the four bacterial strains, whereas compound bearing electron withdrawing group, chloro or fluoro (4’’c & 4’’d) exhibited potential antifungal activity. Compound 4’’d emerged as lead compound. Compound bearing both electron withdrawing (-Cl) and donating (- OH, -OCH3) groups (4’’l) appeared to be the most active against Gram-positive and Gram-negative bacteria, whereas compound bearing only electron donating groups (4’’j) exhibited potential antifungal activity. These compounds could be further derivatized to get even better antimicrobial agents. The data of antimicrobial screening showed the antibacterial and antifungal potential of the pyrazolo-pyridazine derivatives. These compounds could be further derivatized to get even better antimicrobial agents.
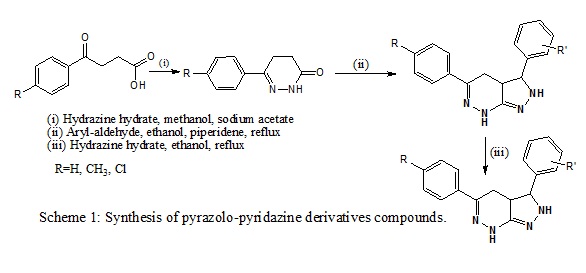
Scheme 1: Synthesis of pyrazolo-pyridazine derivatives compounds.
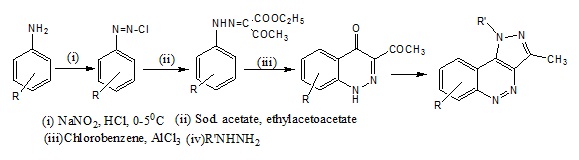
Scheme 2: Synthesis of pyrazolo pyridazine derivatives (5a-f).
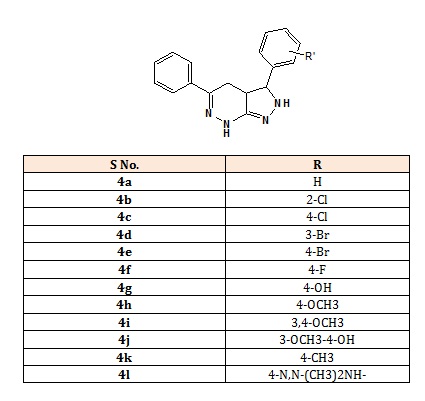
Structure of compounds (4a-4l).
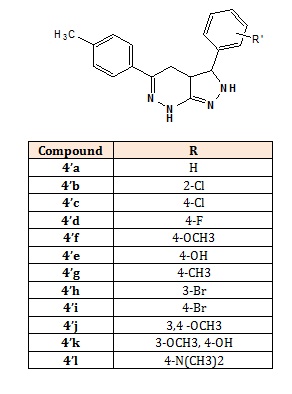
Structures of the compounds (4’a-l).
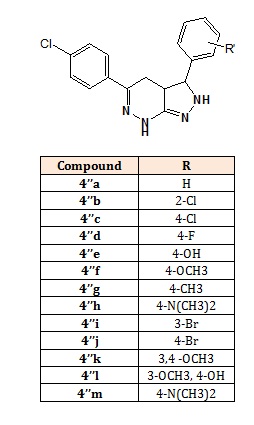
Structure of compounds (4’’a-m).
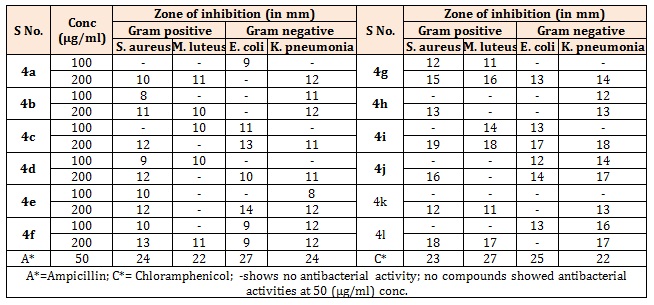
Table 1A: Antibacterial activity of 3,5-disubstituted-phenyl-3,3a, 4,7-tetrahydro-2H-pyrazolo[3,4-c] pyridazine (4a-l).
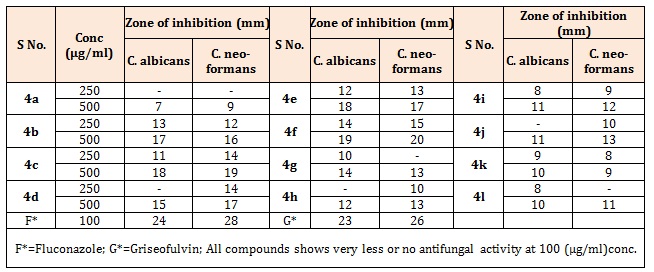
Table 1B: Antifungal activity of 3,5-disubstituted-phenyl-3,3a,4,7-tetrahydro-2H-pyrazolo[3,4-c] pyridazine (4a-l).
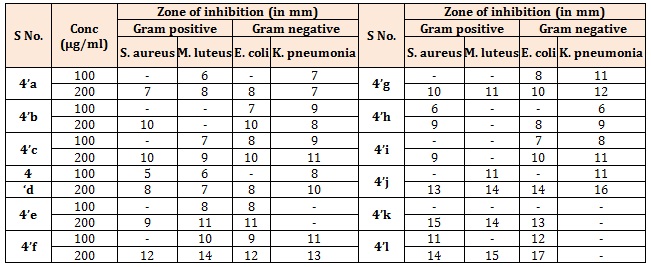
Table 2A: Antibacterial activity of the title compounds (4a-g).
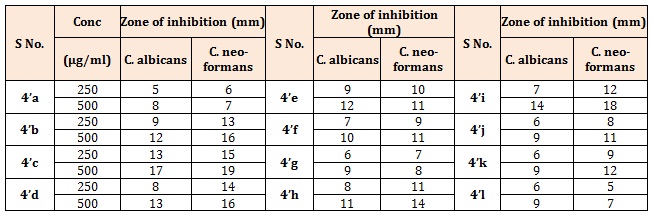
Table 2B: Antifungal activity of the title compounds (4a-g).
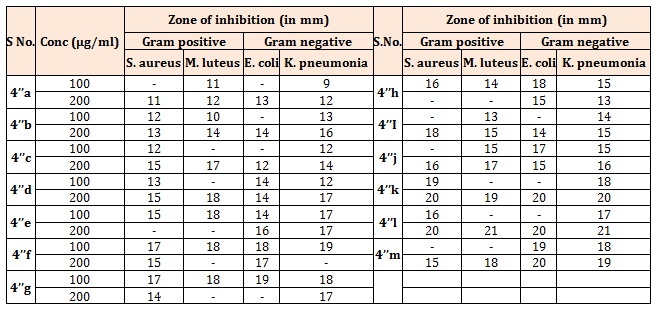
Table 3A: Antibacterial activity of the compounds (4’’a-m).
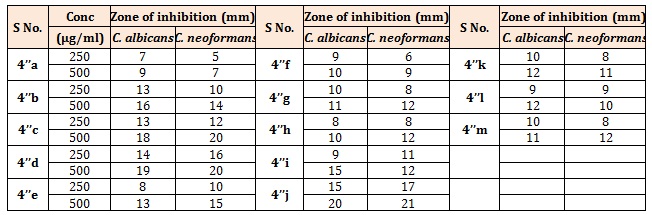
Table 3B: Antifungal activity of the title compounds (4’’a-m).
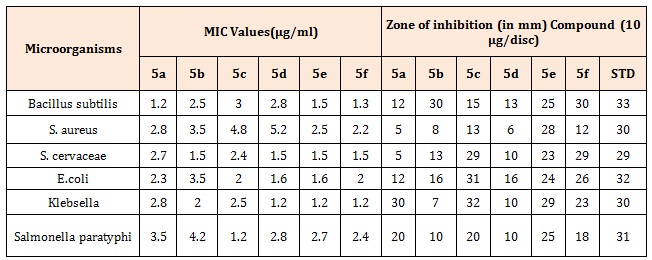
Table 4A: Zone of inhibition for Gram +ve and Gram –ve organisms and Anti-bacterial activity [MIC] of the synthesized compounds by Serial Dilution method.
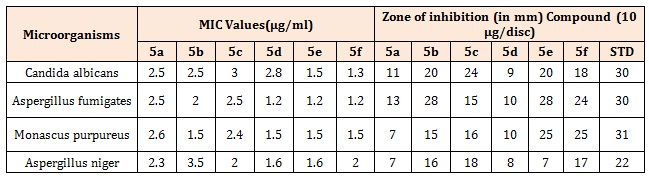
Table 4B: Anti-fungal activity [MIC] of the synthesized compounds by Serial Dilution method and Anti-fungal activity of the synthesized compounds by Disc Diffusion method.
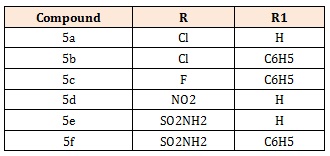
Table 5: Structure of synthesized compounds.
Chat with us on WhatsApp